

Background: Recruiting patients for research is one of the most challenging aspects of a study. Researchers may employ a plethora of methods to recruit patients, including physician referrals, flyers, emails, patient portal messages, social media announcements and cold calls.
Objectives: The objective of this study was to assess the impact of recruitment methods adapted by a remote recruitment process to conduct remote and decentralized research studies. We identified factors associated with successful and efficient screening and recruitment including demographics and social determinants of health.
Methods: The study was conducted within the Excellence Network in Rheumatology (ENRGY) practice-based research network (PBRN). Physicians were presented with information on four studies, and each opted to allow their patients to be contacted. A standard operating procedure was developed in which patients were sent an introductory text briefly describing the study, encouraging participation, and indicating that a remote recruiter would be contacting them. Approximately 10-60 minutes after the text, the remote recruiter called the patient. If patients did not answer, the call was documented and a second call placed 24 hours later. If there was still no response, a second text and call (text occurring 10-60 minutes prior to the call) was placed 7 days later for a total of 5 contacts (2 texts and 3 phone calls). If patients were not reached by the fifth contact, further contact was abandoned. If a patient’s voicemail was reached, messages were left. The two outcomes examined were 1) agreeing to participate (i.e. intent to participate) and 2) enrollment (i.e. actually participating). We analyzed descriptive statistics of patient demographics, enrollment status, and patient contacts. Crude and adjusted logistic regression modeled the odds of enrolling or agreeing to participate in studies.
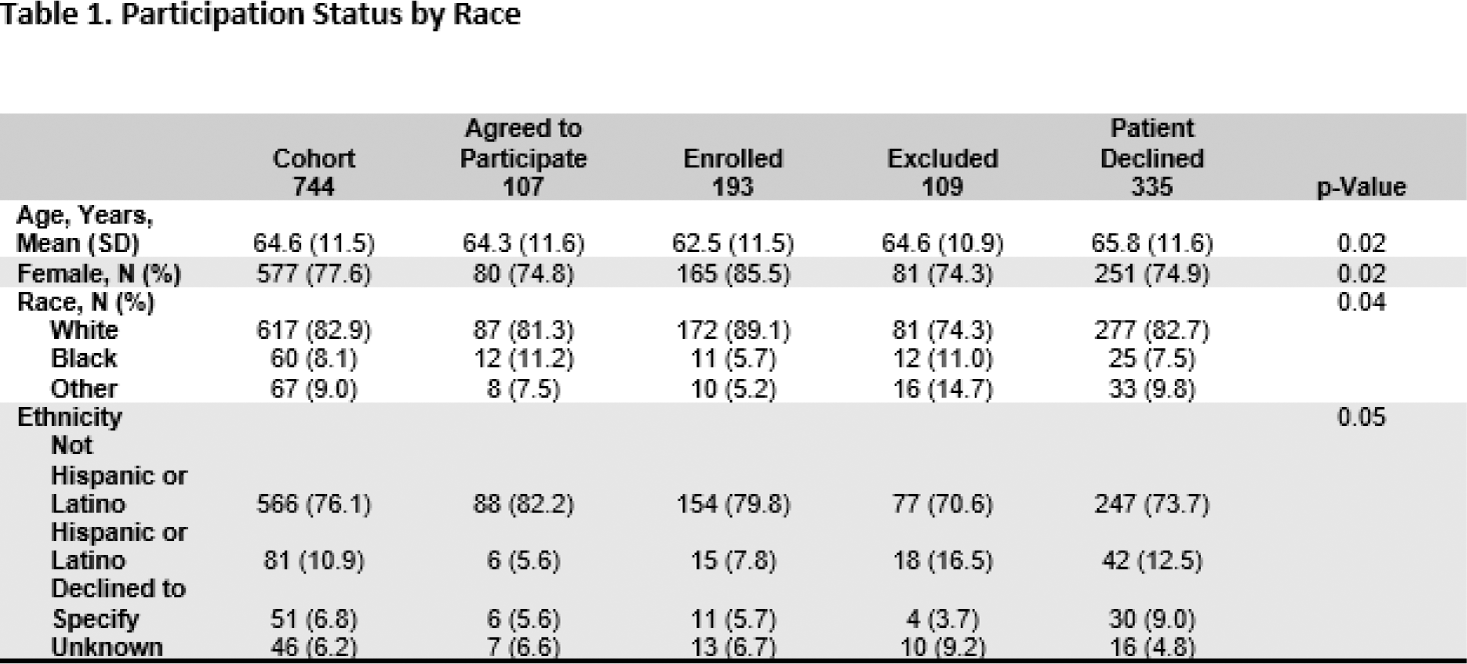
Results: To date, a total of 3,276 patients have been contacted (text or called) at least one time, with 1,097 (34%) patients being reached by the remote recruiter. Of these, 353 patients were reached, but deemed to be ineligible based on study eligibility criteria, leaving 744 eligible for participation. Among these individuals, 107(14%) agreed to participate while on the call, but subsequently did not sign the consent form and enroll in the study; 193 (26%) consented and enrolled; 109 (15%) were excluded, and 335 (45%) declined to participate.
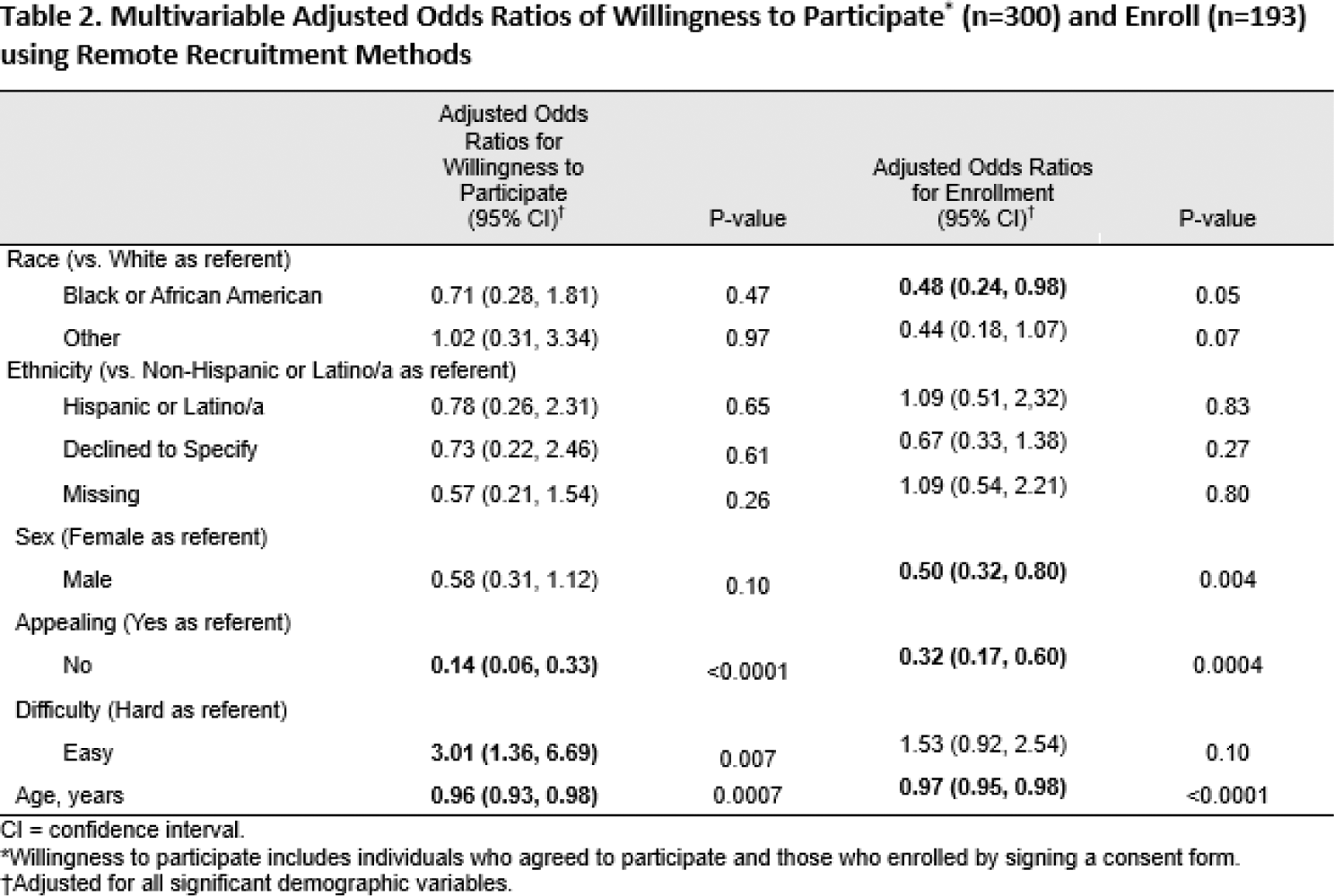
African American patients more likely than White patients to agree to participate (20% vs. 14%), but less likely to consent and enroll (i.e., consent to participate in the study; 18% and 28%, respectively; Table 1). Results from adjusted models indicated that African Americans were 52% less likely to enroll than White populations (aOR: 0.48; 95% CI: 0.24 - 0.98; Table 2).
Furthermore, we found that the added utility of additional calls was greatest at 7 days post the initial call.
Conclusion: Although African American populations indicated willingness to participate in research, they were less likely to consent and enroll in the studies, indicating a need for further research to determine the disconnect between willingness to participate and successful enrollment and consent. Furthermore, the greatest yield of patient “reach” appears to be on the third call occurring 7 days after the first. This seems to indicate that the second call, 24 hours after the first, could be eliminated.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Jeffrey R. Curtis Abbvie, Amgen, Aqtual, Bendcare, BMS, FASTER, GSK, Janssen, Lilly, Moderna, Novartis, Pfizer, Sanofi, Scipher, Setpoint, TNacity Blue Ocean, UCB, Abbvie, Amgen, Aqtual, Bendcare, BMS, FASTER, GSK, Janssen, Lilly, Moderna, Novartis, Pfizer, Sanofi, Scipher, Setpoint, TNacity Blue Ocean, UCB, Abbvie, Amgen, Aqtual, Bendcare, BMS, CorEvitas, GSK, Janssen, Lilly, Moderna, Novartis, Pfizer, Sanofi, Scipher, Setpoint, UCB, Cassie Clinton: None declared, Emily Holladay: None declared, Christian Curtis: None declared, Fenglong Xie: None declared, Patrick Stewart: None declared, Benjamin Nowell: None declared.