

Background: Autoimmune inflammatory rheumatic diseases (AIIRDs) are systemic conditions marked by immune responses against self-antigens, exhibiting diverse clinical manifestations and heterogeneous pathophysiology among patients. Despite this variability, the lack of personalized diagnostics and therapies remains a challenge. Immunophenotyping has been widely used among various experimental methods, offering dynamic and quantitative insight into the immunological status of individual patients.
Objectives: We aimed to analyze the overall immune profiles of patients with SLE, RA, Axial SpA, and Gout, the major diseases of AIIRDs using immunophenotyping.
Methods: The study enrolled 14 patients each with SLE, RA, Axial SpA, and 8 with gout, who met recent ACR/EULAR or ASAS criteria for each disease. Twelve healthy volunteers served as controls. Immunophenotyping of peripheral blood mononuclear cells from both AIIRD patients and healthy controls was conducted using flow cytometry. The panel covered circulating B cells, T cells, dendritic cells, NK cells, NKT cells, MAIT cells, and activation markers such as CD69, PD-1, LAG-3, and Annexin V.
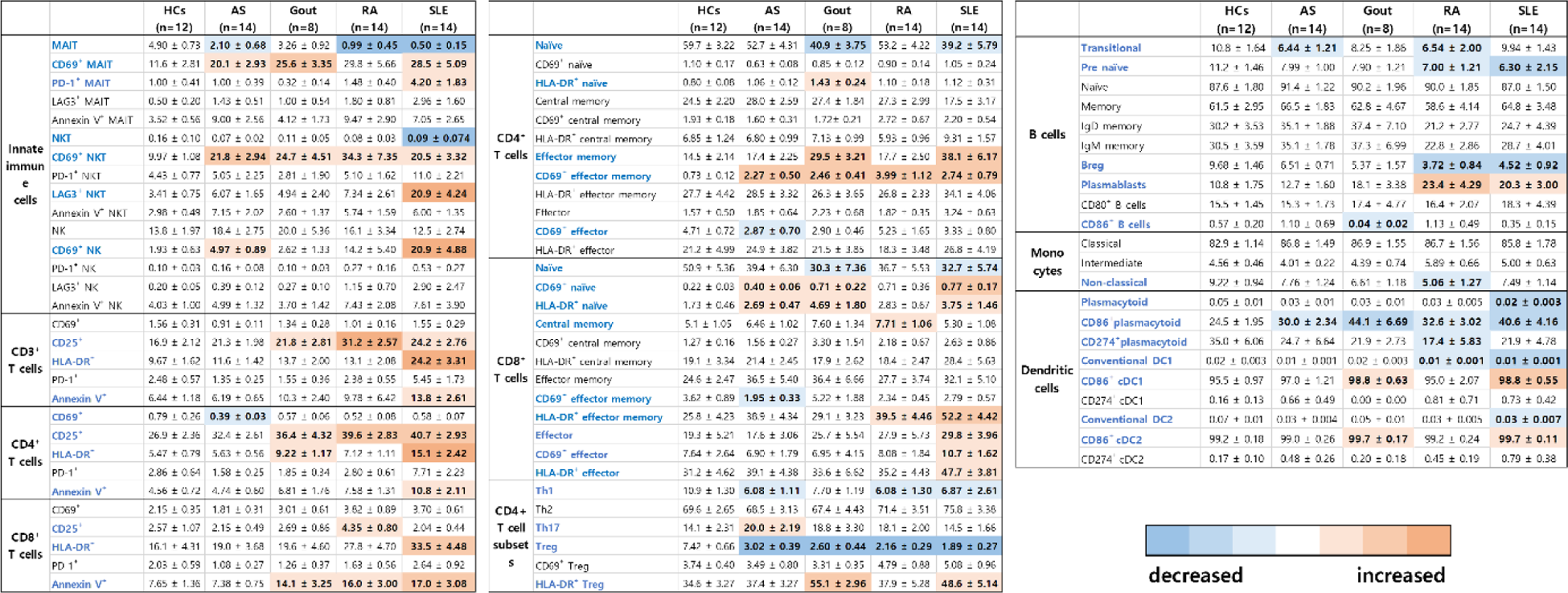
Results: In the analysis of innate and innate-like lymphocytes, Axial SpA and RA patients exhibited a decrease in MAIT cell numbers compared to healthy controls (HCs), while both MAIT and NKT cells were reduced in SLE patients. CD69 expression on MAIT, NKT, and NK cells was elevated in AIIRDs patients compared to HCs, indicating activation of these cells in the AIIRDs condition. Furthermore, CD4+CD69 effector memory cells increased, and Tregs decreased in AIIRDs patients compared to HCs. These findings, combined with the diminished CD86 expression in plasmacytoid dendritic cells in AIIRDs, suggested a regulatory function decrease due to reduced pDC-Treg interaction in AIIRDs patients.
Conclusion: Despite limited clinical data and small patient samples, AIIRD patients exhibit distinct immunologic patterns compared to the healthy population. Further analysis, especially through the application of clustering and regression models, could reveal more detailed immune signatures of these patients and facilitate a more personalized approach to diagnosing and treating AIIRD patients.
REFERENCES: NIL.
Table 1.
Acknowledgements: NIL.
Disclosure of Interests: None declared.