

Background: Counselling patients with rheumatic and musculoskeletal diseases (RMD) in the phase of reproduction, pregnancy and lactation is a challenging task. In women, the potential risk of drug therapy needs to be weighed against the risk of untreated maternal RMD and associated risks for the mother and the child. In men, the safety of antirheumatic drugs with regard to fertility and pregnancy/offspring outcomes needs to be considered.
The first EULAR points to consider (PtC) for the use of antirheumatic drugs before pregnancy, and during pregnancy and lactation published in 2016 have been widely used [1].
Meanwhile, modern treatment approaches have evolved towards a treat-to-target concept to avoid the negative impact of active disease on reproductive function and pregnancy outcomes. Additionally, several relevant data have emerged about antirheumatic drugs in reproduction, pregnancy and lactation.
Objectives: To update the EULAR PtC for use of antirheumatic drugs in reproduction, pregnancy and lactation including additional drugs and adverse outcomes as well as paternal drug safety.
Methods: According to the EULAR standardised operating procedures, the task force involving 27 international members formed six PIO research questions for the systematic literature review (SLR) (detailed protocol registered in PROSPERO, No CRD42022357689).
The result of the SLR was presented to the task force during a consensus meeting in October 2023 to develop this update. A predefined voting process was applied to each overarching principle and statement. Level of evidence and strength of recommendation were assigned, and participants finally provided their level of agreement to each item.
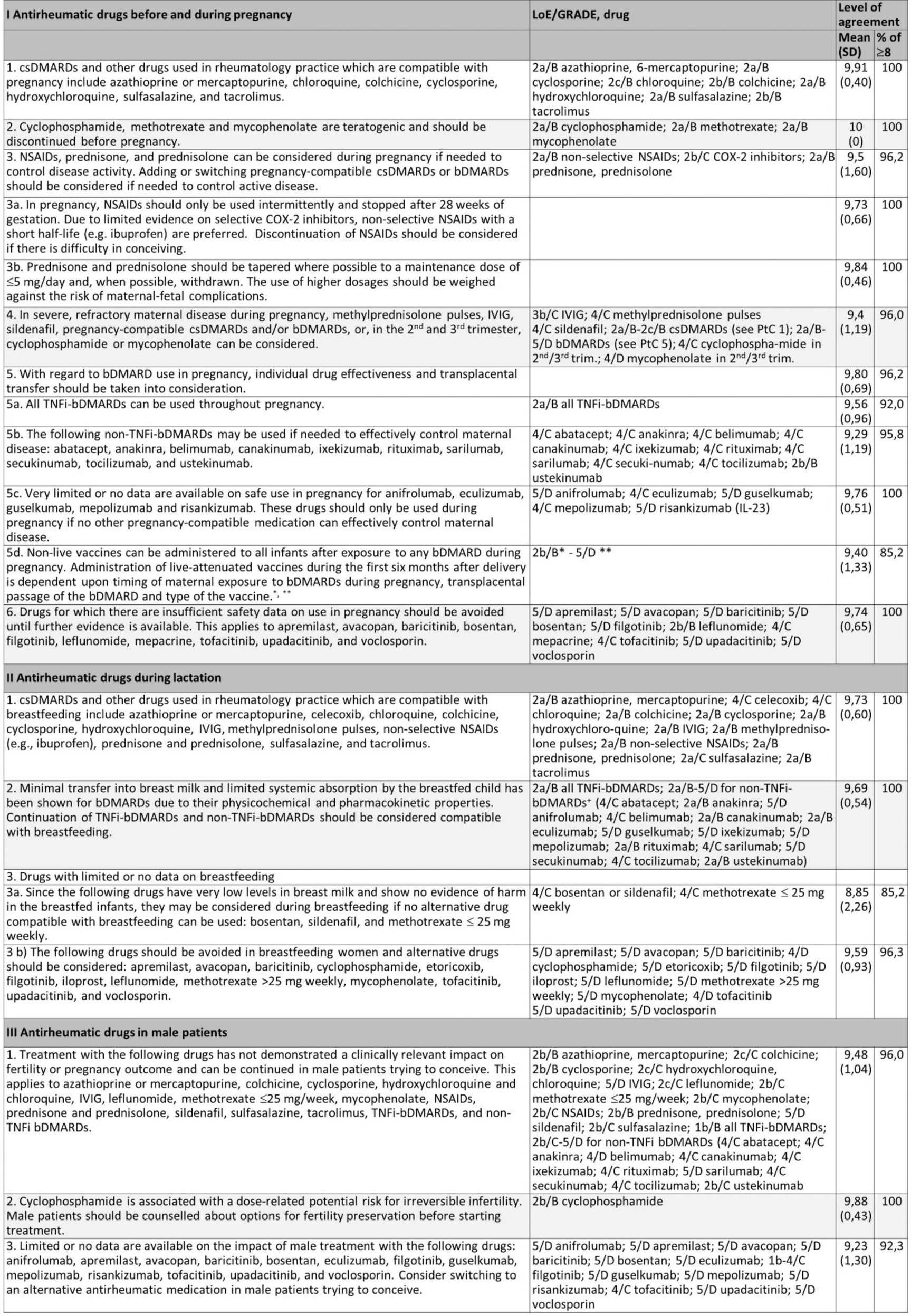
Results: The SLR yielded 8025 articles (2015-2023: 5183; 2000-2015 for outcomes not covered in previous EULAR PtC: 2842), of which 2385 full text articles were reviewed for eligibility. Out of those, 307 were extracted and further analysed. Based on the result of the SLR, the task force developed 5 overarching principles (Table 1), 12 PtC for the use of antirheumatic drugs before and during pregnancy, 4 PtC for the use of antirheumatic drugs in lactation and 3 for the use of antirheumatic drugs in male patients (Table 2).
The current evidence indicates that synthetic DMARDs compatible with pregnancy include azathioprine or mercaptopurine, chloroquine, colchicine, cyclosporine, hydroxychloroquine, sulfasalazine, and tacrolimus. Regarding NSAIDs and glucocorticoids, a more restrictive approach to their use during pregnancy is recommended. Additionally, due to a more favourable risk–benefit profile, the use of bDMARDs, including nonTNFi, is more permissive.
In relation to lactation, compatible drugs include azathioprine or mercaptopurine, celecoxib, chloroquine, colchicine, cyclosporine, hydroxychloroquine, IVIG, methylprednisolone pulses, non-selective NSAIDs (e.g., ibuprofen), prednisone and prednisolone, sulfasalazine, and tacrolimus. A more permissive use is recommended for bDMARDs.
Finally, concerning the use of drugs in men, compatible options include azathioprine or mercaptopurine, colchicine, cyclosporine, hydroxychloroquine and chloroquine, IVIG, leflunomide, methotrexate (≤25 mg/week), mycophenolate, NSAIDs, prednisone and prednisolone, sildenafil, sulfasalazine, tacrolimus, and bDMARDs. Recommendations on infant vaccinations are provided.
Conclusion: The updated EULAR PtC provide consensus guidance and will help to improve the management of patients during the phase of reproduction, pregnancy and lactation.
REFERENCES: [1] Gotestam Skorpen, 2016, DOI: 10.1136/annrheumdis-2015-208840.
Table 1. EULAR overarching principles for the use of antirheumatic drugs in reproduction, pregnancy and lactation

Table 2. EULAR points to consider for use of antirheumatic drugs in reproduction, pregnancy and lactation
Acknowledgements: NIL.
Disclosure of Interests: Frauke Förger Mepha, Roche, UCB Pharma, MSD, UCB Pharma, GSK, Andrea Pluma Sanjurjo: None declared, Linda Rüegg: None declared, Sabrina Hamroun: None declared, Irene Cecchi: None declared, Luis Fernando Perez: None declared, Philip Anderson: None declared, Laura Andreoli: None declared, Sara Badreh: None declared, Vladimira Boyadzhieva: None declared, Christina Chambers: None declared, Nathalie Costedoat-Chalumeau: None declared, Radboud J.E.M. Dolhain: None declared, Rebecca Fischer-Betz: None declared, Ian Giles: None declared, Carina Götestam Skorpen: None declared, Maria Hoeltzenbein: None declared, Francesca Marchiori: None declared, Karoline Mayer-Pickel: None declared, Anna Moltó: None declared, Catherine Nelson-Piercy: None declared, Ole Haagen Nielsen: None declared, Angela Tincani: None declared, Marianne Wallenius: None declared, Astrid Zbinden: None declared, Yvette Meissner Lilly, Pfizer, Axel Finckh: None declared.