

Background: Patients are essential stakeholders in medical research. The 2011 EULAR recommendations for Patient Research Partner (PRP) involvement in rheumatology research were critical in promoting PRP involvement;[1] since then, many studies concerning PRP involvement have been published.[2]
Objectives: The aim was to develop updated EULAR recommendations for PRPs in rheumatology research.
Methods: In accordance with the EULAR Standardized Operational Procedures, a taskforce comprising 13 researchers, 2 health professionals in rheumatology and 10 PRPs was convened. The process included an online taskforce meeting, a systematic literature review, and an in-person second taskforce meeting in 2023 to formulate overarching principles (OAPs) and recommendations. The strength of the recommendations was assessed through their evidence-based grade and taskforce members indicated their level of agreement (0-10 scale).
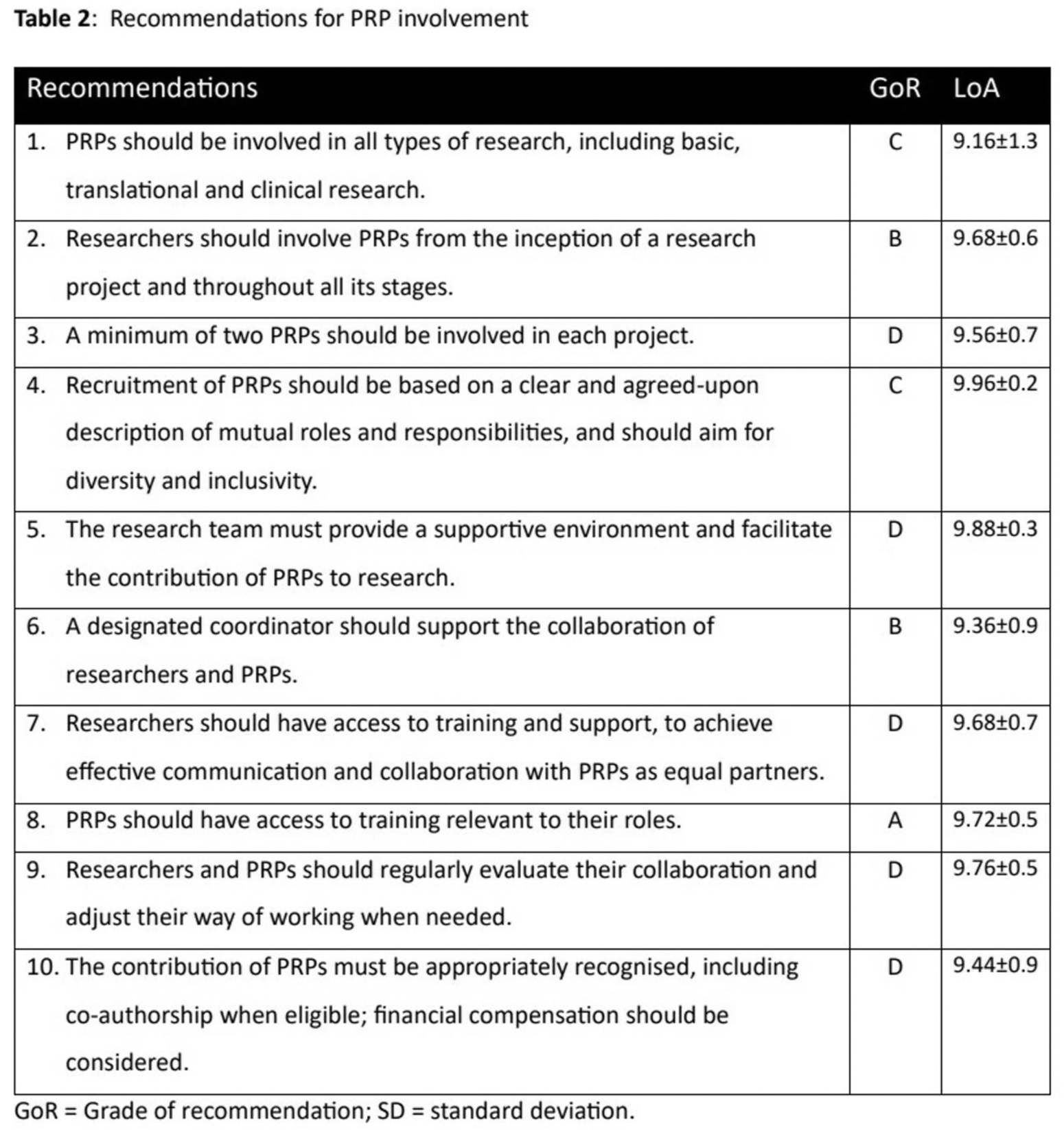
Results: The task force developed five new OAPs (Table 1), updated seven existing recommendations and formulated three new recommendations (Table 2). The OAPs address the definition of PRP, the contribution of PRPs, the role of informal caregivers, the added value of PRPs, and the importance of trust and communication in collaborative research efforts. The updated recommendations cover topics such as research type (including basic and translational research), involvement from the research project’s inception, the recommended number of PRPs per project, and the support, training and acknowledgement of PRPs. New recommendations concern the benefits of support and guidance for researchers, the need for regular monitoring and evaluation of the patient-researcher collaboration, and the role of a designated coordinator to facilitate collaboration. Strengths of the recommendations varied (range, A-D); agreements within the taskforce were high (range 9.2-10).
Conclusion: We updated the EULAR recommendations for PRP involvement in rheumatology research, now more substantially based on recent evidence. The recommendations cover practical issues for the involvement of PRPs in research and are in line with EULAR’s principles of inclusive and collaborative research. These recommendations should serve as a guide for researchers and PRPs to strengthen the involvement of PRPs in rheumatology research, ultimately resulting in better research.
REFERENCES: [1] de Wit MP, Berlo SE, Aanerud GJ, Aletaha D, Bijlsma JW, Croucher L, et al. European League Against Rheumatism recommendations for the inclusion of patient representatives in scientific projects. Ann Rheum Dis. 2011;70(5):722-6.
[2] Aouad K, de Wit M, Elhai M, Benavent D, Bertheussen H, Zabalan C, et al. Barriers and Strategies to enhance Patient Research Partner Involvement in Rheumatology research: a systematic literature review informing the 2023 updated EULAR recommendations for the involvement of patient research partners in scientific projects (in review) 2023.

Acknowledgements: Funded by EULAR (RES005).
Disclosure of Interests: None declared.