

Background: Nintedanib is an intracellular inhibitor of multiple tyrosine kinases. It is the current standard treatment for idiopathic pulmonary fibrosis (IPF), Systemic Sclerosis-associated pulmonary fibrosis and progressive pulmonary fibrosis. Nintedanib is associated with a significant reduction in the forced vital capacity (FVC) decline in pivotal trials. More than 60% of patients reported adverse effects (AE) like nausea, diarrhea, weight loss and liver toxicity, leading to drug discontinuation in 20% of patients. Low-performance status and low body mass index (BMI) were identified as possible risk factors for the AE increased incidence. Managing antifibrotic treatment and predicting and minimizing nintedanib AE is the cornerstone for the appropriate treatment of pulmonary fibrotic disease.
Objectives: Reporting the experience of a multidisciplinary interstitial lung disease (ILD) team in treating fibrotic lung disease with nintedanib, analyzing possible risk factors for nintedanib dose reduction or discontinuation.
Methods: Baseline characteristics and treatment outcomes of patients on nintedanib with fibrotic ILD, diagnosed between January 2015 and October 2023, were retrospectively collected. Statistical analysis was realized using one-way ANOVA and Kruskal-Wallis tests. Logistic regression models were built to assess which baseline characteristics contribute to variation between treatment groups (continuous full dose vs composite variable of dose reduction/discontinuation), controlling for potential confounding factors. Drug survival analysis was conducted using Cox regression and Kaplan-Meier curves.
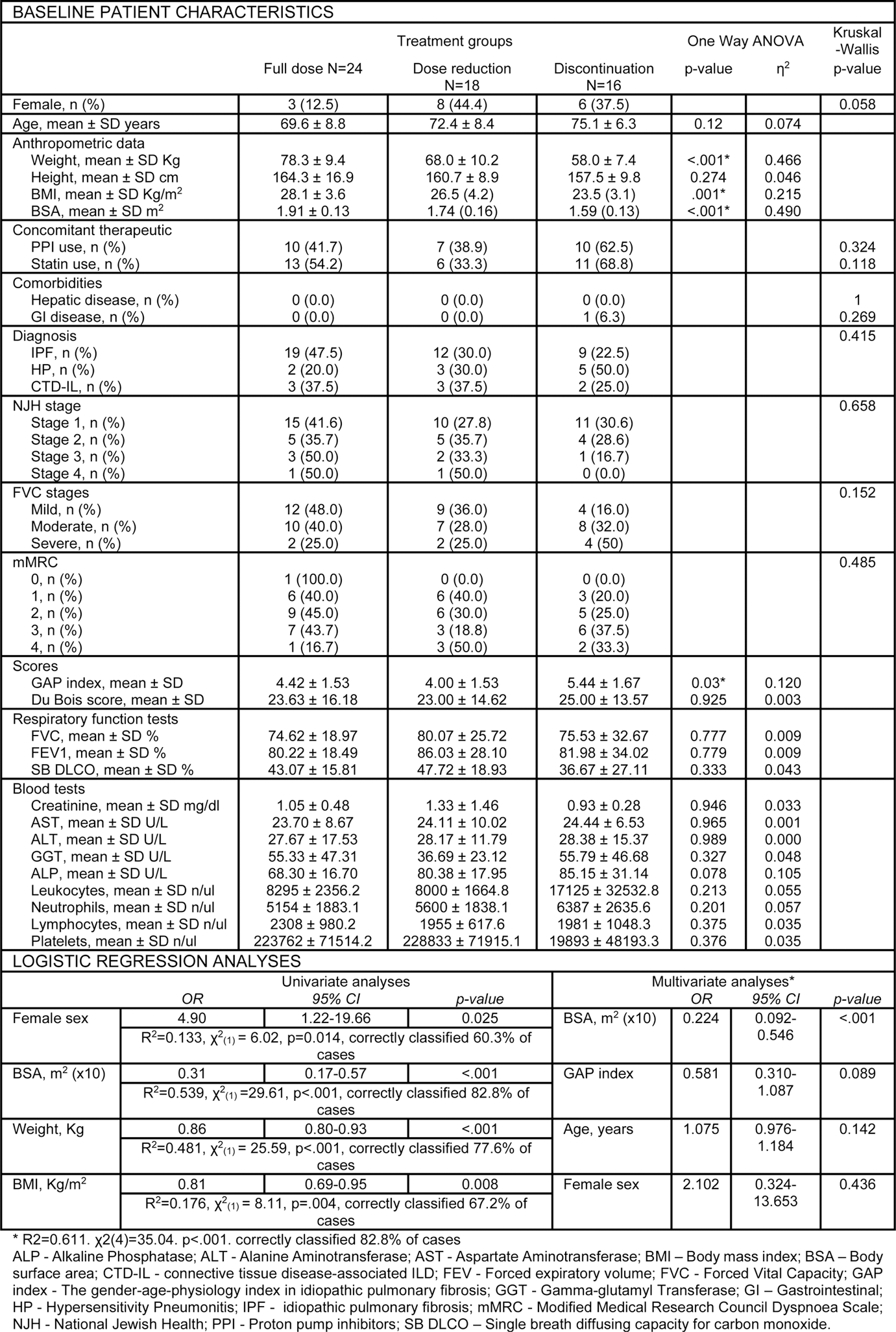
Results: Among the 58 patients on nintedanib, 40 (69%) had IPF, 10 (17.2%) had Hypersensitivity Pneumonitis and 8 (13.8%) had connective tissue disease-associated ILD. The mean age was 71.98 ± 8.25 years and 17 (29.3%) were females. At the last visit, 24 patients (41.4%) were on full dose nintedanib; 18 (31.0%) required dose reduction and 16 (27.6%) discontinued treatment due to AE (diarrhea, nausea, vomiting or hepatotoxicity). Weight, BMI, and body surface area (BSA) exhibited an inverse relationship with the treatment discontinuation likelihood in post-hoc Tukey HSD analysis. The detailed baseline characteristics are presented in Table 1. Univariate logistic regression identified BSA (OR 0.31, p<0.001), weight (OR 0.86, p<.001), BMI (OR 0.81, p=0.008) and female sex (OR 4.90, p=0.025) as potential predictors for nintedanib dose reduction or discontinuation. Multivariate logistic regression analysis (Table 1) revealed that baseline BSA (adjusted OR 0.22, p<.001) was an independent risk factor for nintedanib dose reduction or discontinuation. The ROC curve performed revealed a cutoff value of BSA ≤ 1.73m 2 (area under the curve (AUC) 0.880, p<.001) exhibiting a sensitivity of 73% and specificity of 91.7%. Individuals with BSA ≤1.73m 2 had reduced one-year survival rates under full-dose treatment when compared with those whose BSA >1.73m 2 (Log Rank test χ2 (1) = 26.55, p<.001), with a median full-dose survival of 171 days (Figure 1). The adjusted hazard ratio to full dose dropout was 5.16 (p<.001).
Table 1. Descriptive statistics of baseline variables and univariate and multivariate logistic regression models exploring the impact of each variable in the need for nintedanib dose reduction or discontinuation.
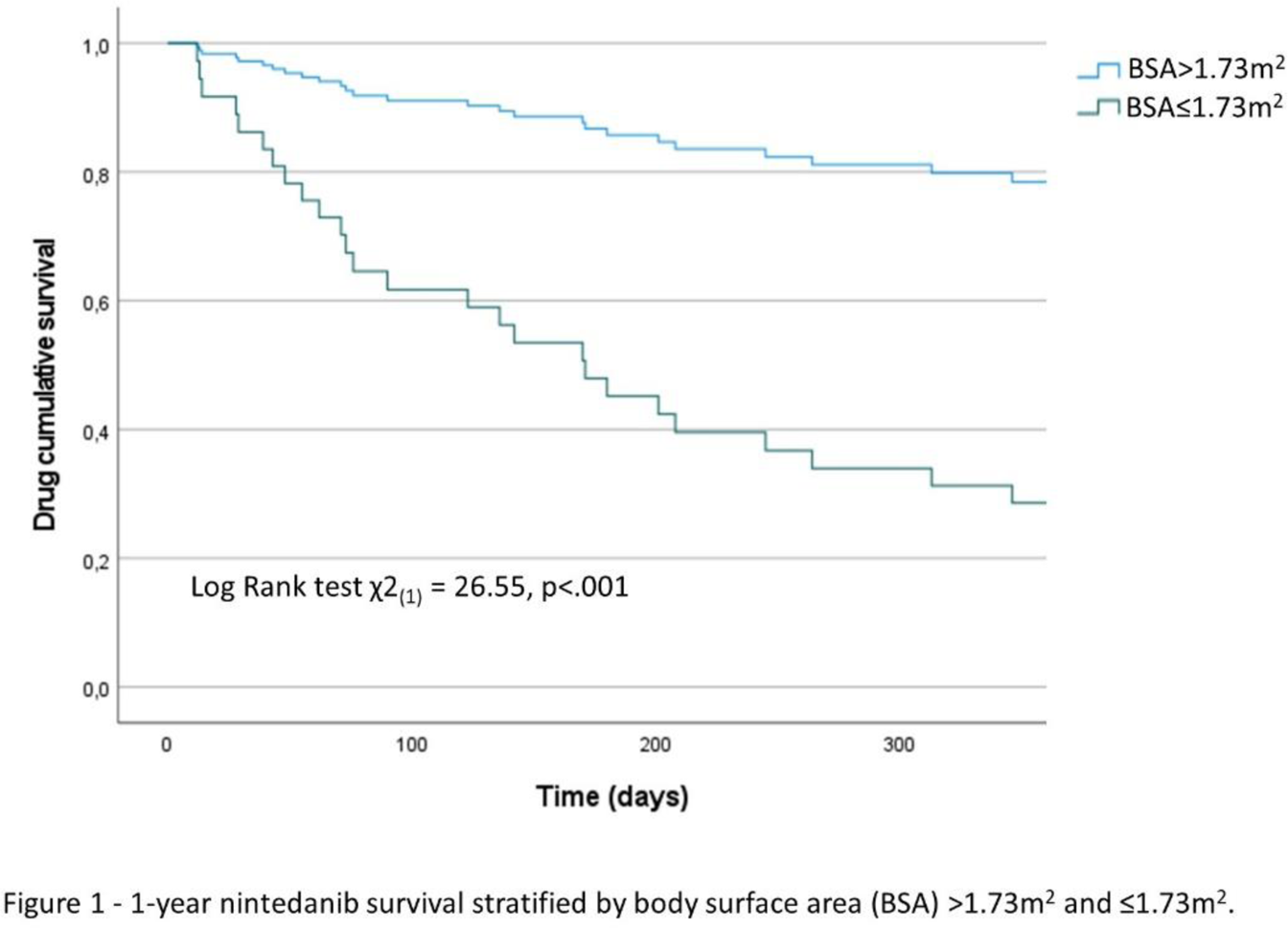
Conclusion: These results imply a strong association between lower BSA and the early onset of intolerance or AE. Consistent with the findings of Ikeda S. et al., all 8 patients who required nintedanib dose reduction or discontinuation due to hepatotoxicity had a BSA <1.6m 2 . This implies a proportional relationship, supporting the results that lower BSA increases the likelihood of AE, particularly gastrointestinal, leading to nintedanib dose reduction or discontinuation. Furthermore, the occurrence of these AE occurs early during drug administration. In addition to our study, other researchers had identified easily assessable factors applicable in clinical practice (BSA, BMI, FVC and Performance status) associated with reduced nintedanib tolerability. In conclusion, prescribers of nintedanib must recognize these factors and drive regular clinical and analytical monitoring. Efforts should be made to ensure the drug’s safety and tolerability, preventing the progression of pulmonary fibrosis.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.