

Background: Pooled safety data have been reported for secukinumab (SEC) administration in patients with psoriasis (PsO), psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA). [1,2]
Objectives: Here, we report safety profile of SEC after extensive patient exposure in clinical trials from a larger pool of patients and including more studies than previously published. [3]
Methods: The pooled safety analysis included 47 Phase II/III/IV clinical trials (PsO: 30; PsA: 9; axSpA [including ankylosing spondylitis {AS} and non-radiographic axial spondyloarthritis {nr-axSpA}]: 8 trials) with patients who had received ≥1 dose of subcutaneous SEC 150 mg and/or 300 mg for ≥16 weeks (cut-off: June 2022). Adverse events (AEs) were reported as exposure-adjusted incidence rates (EAIRs)/100 patient-years (PYs).
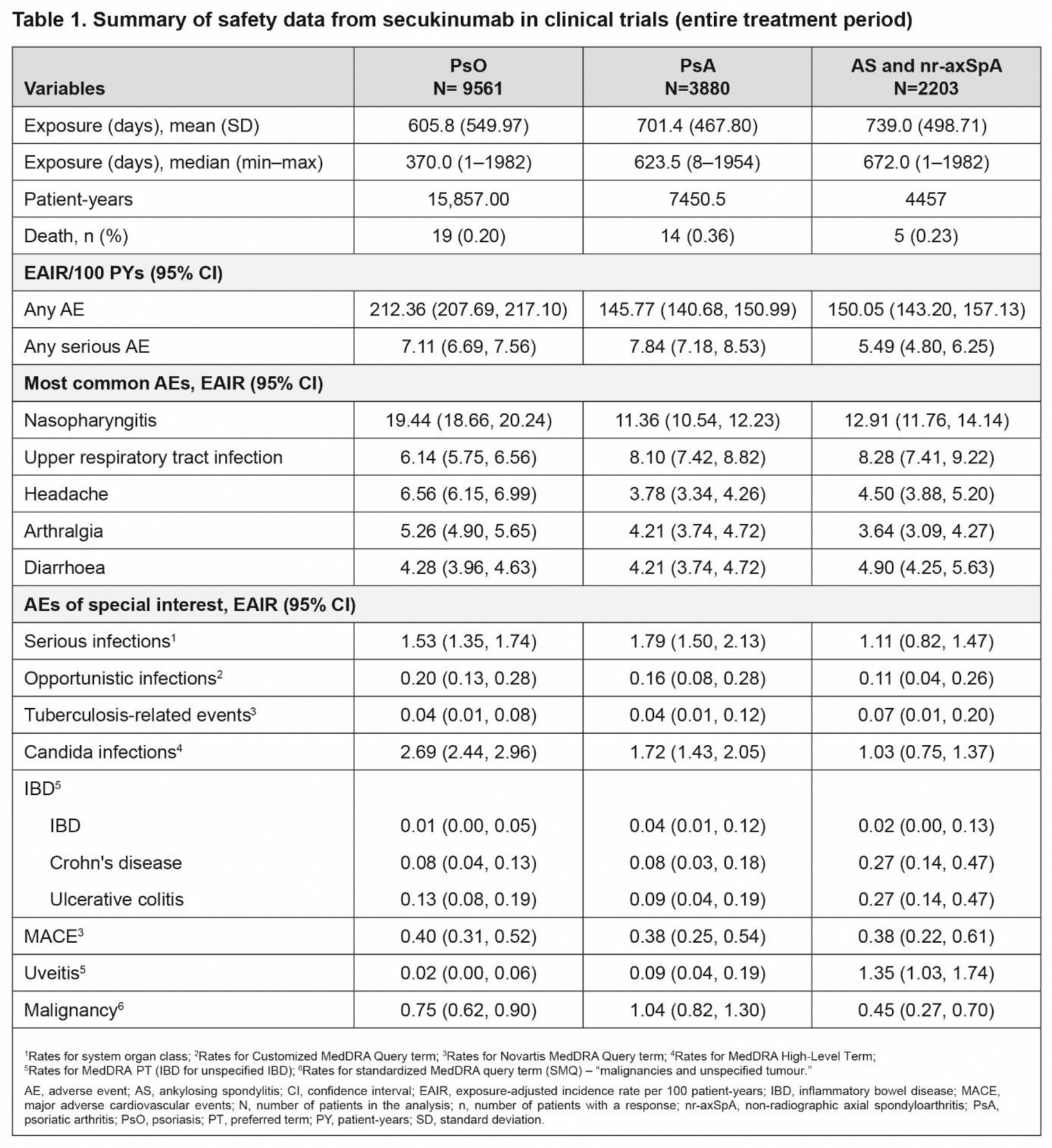
Results: A total of 15,644 patients (PsO [N=9561], PsA [N=3880] and axSpA [N=2203]) were included in the analysis. The most frequent AEs reported across all indications were nasopharyngitis (EAIR [95% confidence interval {CI}]: PsO, 19.44 [18.66, 20.24]; PsA, 11.36 [10.54, 12.23]; axSpA, 12.91 [11.76, 14.14]) and upper respiratory tract infection (EAIR [95% CI]: PsO, 6.14 [5.75, 6.56]; PsA, 8.10 [7.42, 8.82]; axSpA, 8.28 [7.41, 9.22]). EAIRs/100 PYs for inflammatory bowel disease, malignancies and major adverse cardiovascular events remained low across all indications. The EAIRs/100 PYs for AEs of special interest are reported in Table 1.
Conclusion: This pooled safety data analysis of 47 Phase II/III/IV clinical trials demonstrates that secukinumab is well tolerated in patients with PsO, PsA and axSpA, and shows no new signals with longer-term follow-up compared to published studies. 1,2
REFERENCES: [1] Deodhar A, et al. Arthritis Res Ther . 2019;21:111.
[2] Deodhar A, et al. Ann Rheum Dis. 2020;79:722.
[3] Gottlieb AB, et al. Acta Derm Venereol . 2022;102:adv00698.
Acknowledgements: Medical writing assistance was provided by Ishita Guha Thakurta and Divya Chandrashekhar (Novartis Healthcare Pvt Ltd, Hyderabad) which was funded by Novartis Pharma AG, Basel, Switzerland in accordance with the Good Publication Practice (GPP4) guidelines (
Disclosure of Interests: Atul Deodhar Received speaker honoraria from AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, Received honoraria for consulting from AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, Received research grants from AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, Iain B. Mc Innes Received speaker honoraria from AbbVie, Amgen, Bristol Myers Squibb, Cabaletta, Celgene, Compugen, Janssen, Lilly, Moonlake, Novartis, Pfizer, and UCB, Received consultation fees from AbbVie, Amgen, Bristol Myers Squibb, Cabaletta, Celgene, Compugen, Janssen, Lilly, Moonlake, Novartis, Pfizer, and UCB. Board member: Evelo, Received research grant from AbbVie, Amgen, Bristol Myers Squibb, Cabaletta, Celgene, Compugen, Janssen, Lilly, Moonlake, Novartis, Pfizer, and UCB, Xenofon Baraliakos Speaker’s bureau: AbbVie, Bristol Myers Squibb, Celgene, Chugai, Merck, Novartis, Pfizer, and UCB, Consultant for AbbVie, Bristol Myers Squibb, Celgene, Chugai, Merck, Novartis, Pfizer, and UCB, Received research grant from AbbVie, Bristol Myers Squibb, Celgene, Chugai, Merck, Novartis, Pfizer, and UCB, Alice B Gottlieb Honoraria as an advisory board member and consultant for Amgen, Anaptyps Bio, Avotres Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Dice Therapeutics, Eli Lilly, Highlights Therapeutics, Janssen, Novartis, Sanofi, UCB, and Xbiotech, Received research or educational grants from Anaptyps Bio, Highlights Therapeutics, Moonlake Immunotherapeutics AG, Novartis, Bristol Myers Squibb, and UCB Pharma, (all paid to Mount Sinai School of Medicine), Uta Kiltz Received speaker honoraria from AbbVie, Biocad, Chugai, Eli Lilly, Grünenthal, Janssen, MSD, Novartis, Pfizer, Roche, and UCB, Received consultancy honoraria from AbbVie, Biocad, Chugai, Eli Lilly, Grünenthal, Janssen, MSD, Novartis, Pfizer, Roche, and UCB, Received unrestricted grant for research from Abbvie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, and Pfizer, Stefan Schreiber Received consultation fees from AbbVie, Arena, Bristol Myers Squibb, Biogen, Celltrion, Celgene, Falk, Fresenius, Gilead, IMAB, Janssen, MSD, Mylan, Pfizer, Protagonist, Provention Bio, Takeda, and Theravance, Braja Gopal Sahoo Employee of Novartis, Weibin Bao Owns stocks of Novartis, Employee of Novartis, Luminita Pricop Owns stocks of Novartis, Employee of Novartis, Corine Gaillez Owns shares of Novartis and Bristol Myers Squibb, Employee of Novartis, Victor Dong Owns stocks of Novartis, Employee of Novartis, Philip J. Mease Speakers bureau: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Crescendo Bioscience, Genentech, Janssen, Eli Lilly, Merck, Novartis, Pfizer, and UCB, Consultant for: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Crescendo Bioscience, Genentech, Janssen, Eli Lilly, Merck, Novartis, Pfizer, and UCB, Received Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Crescendo Bioscience, Genentech, Janssen, Eli Lilly, Merck, Novartis, Pfizer and UCB.