

Background: Rheumatoid Arthritis (RA) patients and those with other autoimmune diseases were excluded from most clinical trials of immune checkpoint inhibitors (ICI) for fear they might experience excessive immune related adverse events (irAEs). We previously showed that overall survival (OS) is worse in ICI-treated RA patients with non-small cell lung cancer (NSCLC) compared to non-RA patients. [1] IrAEs might explain this comparatively worse OS.
Objectives: Among patients who have or do not have RA, to investigate the relationship between new irAE occurrence and overall survival (OS) among ICI-treated NSCLC patients.
Methods: We used a curated Medicare claims dataset that consists of a 100% sample of patients with pre-existing RA. Patients ≥ 66 years of age with a diagnosis of RA (2+ claims, 30 days apart from a rheumatologist) and NSCLC initiating nivolumab, pembrolizumab or atezolizumab (2+ claims from an oncologist) were included. Non-RA patients with NSCLC who were initiating ICI were identified from the 5% Medicare random sample; patients were excluded if they had a prior RA diagnosis or past DMARD use. ICI use was identified using Healthcare Common Procedure Coding System (HCPS) codes. IrAEs were defined using a relevant ICD 9 or ICD 10 diagnosis code and were grouped into 6 categories: dermatologic, hepatitis, myalgia/myositis, pneumonitis, arthralgia/arthritis, and colitis.
Both 90- and 180-day landmark analyses were performed, and Kaplan Meier (KM) curves and Cox proportional hazard models were created to measure OS from first ICI initiation. Models were adjusted for age, gender, race, ethnicity, interstitial lung disease, chronic obstructive pulmonary disease, prior radiation, year of ICI initiation, modified Elixhauser score, first- or second-line ICI, and receiving ICI with or without chemotherapy. Schoenfeld residuals were used to assess the proportional hazards assumption. Patients were followed through 12/31/2019.
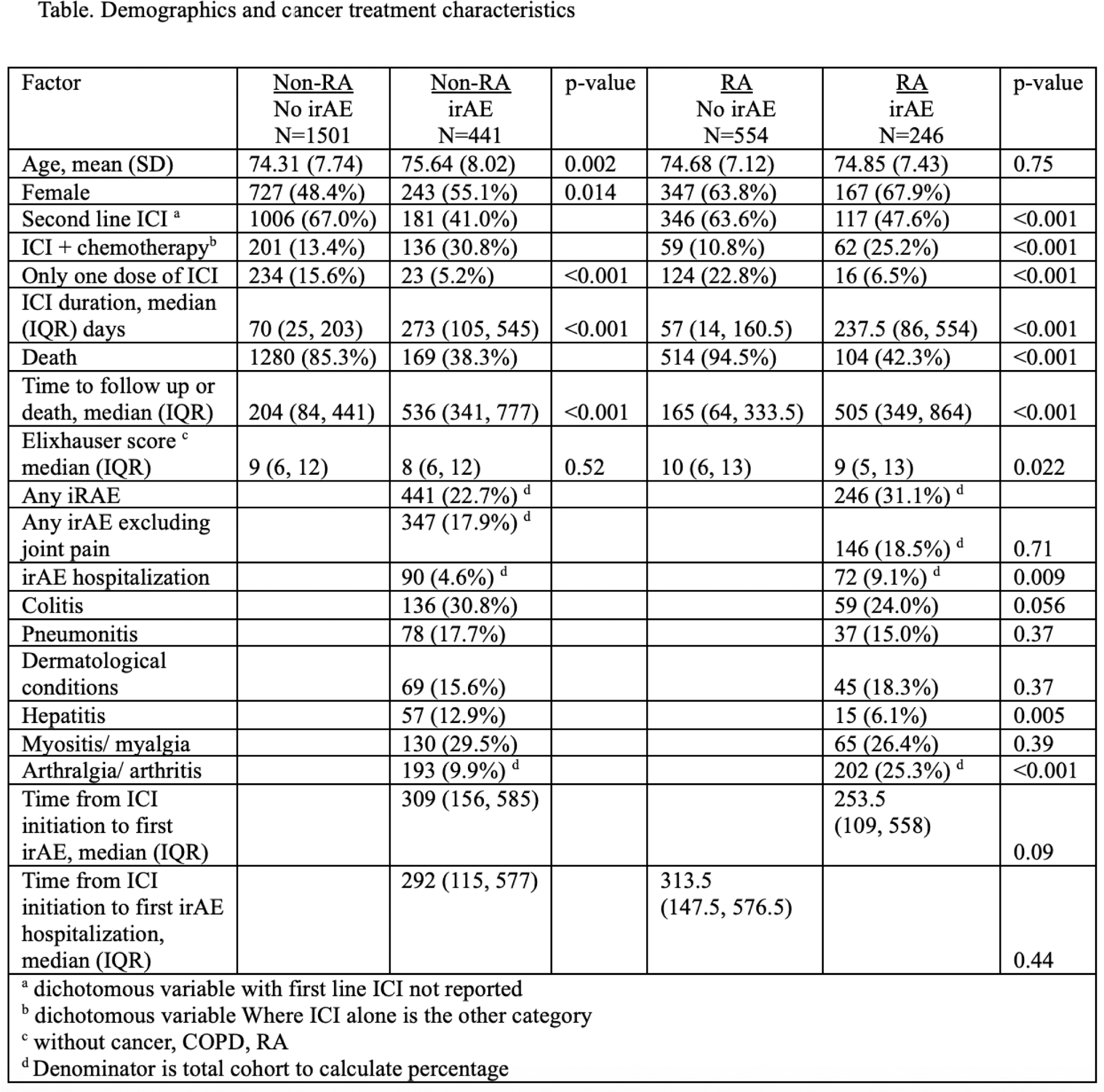
Results: IrAE occurred in 246 (31%) RA patients and 441 (23%) non-RA (p value <0.001) at a median [IQR] of 309 [156,585] and 253 [109,558] days after ICI initiation (Table 1). RA patients also had more claims for irAEs than non-RA patients, median (IQR) 6 (3, 11) versus 3 (1, 6) p <0.001). RA patients also had more hospitalizations associated with an irAE diagnosis (9.1% vs 4.6%). RA patients experienced more arthralgia/arthritis as an irAE (25% vs 10%, p<0.001). Omitting arthralgia/arthritis, the percent of patients experiencing an irAE among RA versus non-RA patients was 18.5% vs 17.9%) (p=0.71).
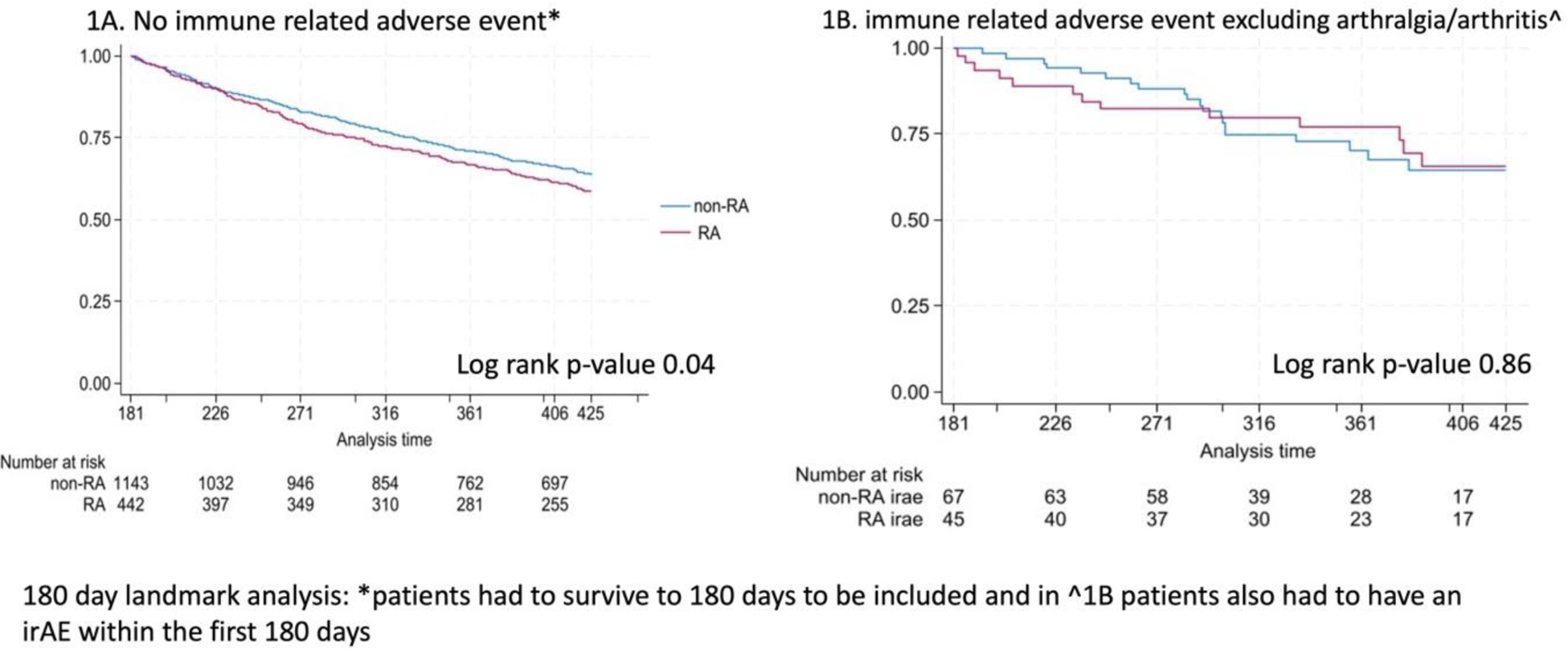
In an analysis of patients who survived but did not experience irAE before day 180 (Figure 1A), KM curve demonstrates worse OS in RA compared to non-RA patients (log rank p-value 0.04). In contrast, among patients who survived to day 180 and experienced an irAE (excluding arthralgia) before that date, there was no association with OS between RA and non-RA patients (log rank p-value 0.42) (Figure 1B).
In a 180-day landmark adjusted Cox model, there was no association between developing an irAE and OS among RA patients, HR 0.71, (95%CI 0.44, 1.15), nor among non-RA patients, HR 1.26, (95%CI 0.82, 1.93). Excluding arthralgia, there was no association between irAE and OS in either RA (HR 0.57, 95%CI 0.31, 1.05) or non-RA patients (HR 1.12 95% CI 0.70, 1.79). A 90-day landmark adjusted model, found no association between developing an irAE and OS among RA patients, HR 0.69, (95%CI 0.40, 1.18). Non-RA patients with an irAE had worse OS, HR 1.68, (95%CI 1.10, 2.56). Excluding arthralgia, there was no association between irAE and OS in either RA (HR 0.65, 95%CI 0.35, 1.22) or non-RA patients (HR 1.49, 95%CI 0.94, 2.36).
Conclusion: OS is worse in RA vs non-RA patients with NSCLC, but only among patients without an irAE. There was no association between developing an irAE and OS among RA patients. In contrast, OS was worse among non-RA patients experiencing an irAE before day 90. Future studies are needed to evaluate the relationship between DMARD use, irAE and OS in patients with RA.
REFERENCES: [1] Jannat-Khah D. ACR Convergence abstract 0967.
Kaplan Meier curves for 180 landmark analysis among patients with and without an immune related adverse event
Acknowledgements: Support for this project came from the Hospital for Special Surgery’s Department of Medicine Discovery Grant. Dr. Curtis is supported by NIAMS P30 AR072583.
Disclosure of Interests: Deanna Jannat-Khah I own shares of AstraZeneca, Fenglong Xie: None declared, Ashish Saxena Consultant for Galvanize Therapeutics, Research funding from AstraZeneca, Jeffrey R Curtis: None declared, Anne Bass: None declared.