

Background: Rheumatic? is an online symptom checker developed collaboratively by designers, engineers, clinical experts and patients. It is designed to identify individuals at high risk of developing rheumatic diseases and patients with immune-mediated rheumatic diseases (IRD). After completion of the questionnaire, Rheumatic? gives a weighted score based on expert assessments of the clinical significance of the answers [1] . A previous retrospective study has demonstrated the potential to distinguish IRD from osteoarthritis(OA) and gout, where clinicians filled out the questionnaire based on the medical notes [2] . In order to use a questionnaire in real life, it should be tested on real life participants, as they may describe their symptoms differently than experts.
In order to evaluate the performance of the current Rheumatic? score, to differentiate between IRDs and non-IRDs we conducted a prospective real-world study in the Netherlands and assessed the differences in Rheumatic? total scores between individuals diagnosed with IRD and non-IRD.
Objectives: Examine the difference in scores of Rheumatic? between IRD (Rheumatoid arthritis, Psoriatic arthritis, Myositis, Sjogren’s syndrome, SLE, Spondyloarthritis and Polymyalgia rheumatica) and non-IRD(OA, Gout, Fibromyalgia and other) diagnoses.
Methods: We analyzed data submitted by Dutch participants recruited via multiple channels between July 2021 and July 2022. After completing the online questionnaire Rheumatic? participants received four follow-up surveys at 1 week, 3, 6 and 12 months asking, among other things, for previous and novel diagnoses. The theoretical total score for the participant was determined using the current, expert developed, risk assessment model post collection of all data [1,2] .
We used t-test and analysis of variance (ANOVA) to compared the observed differences in mean and median of total scores among these groups.
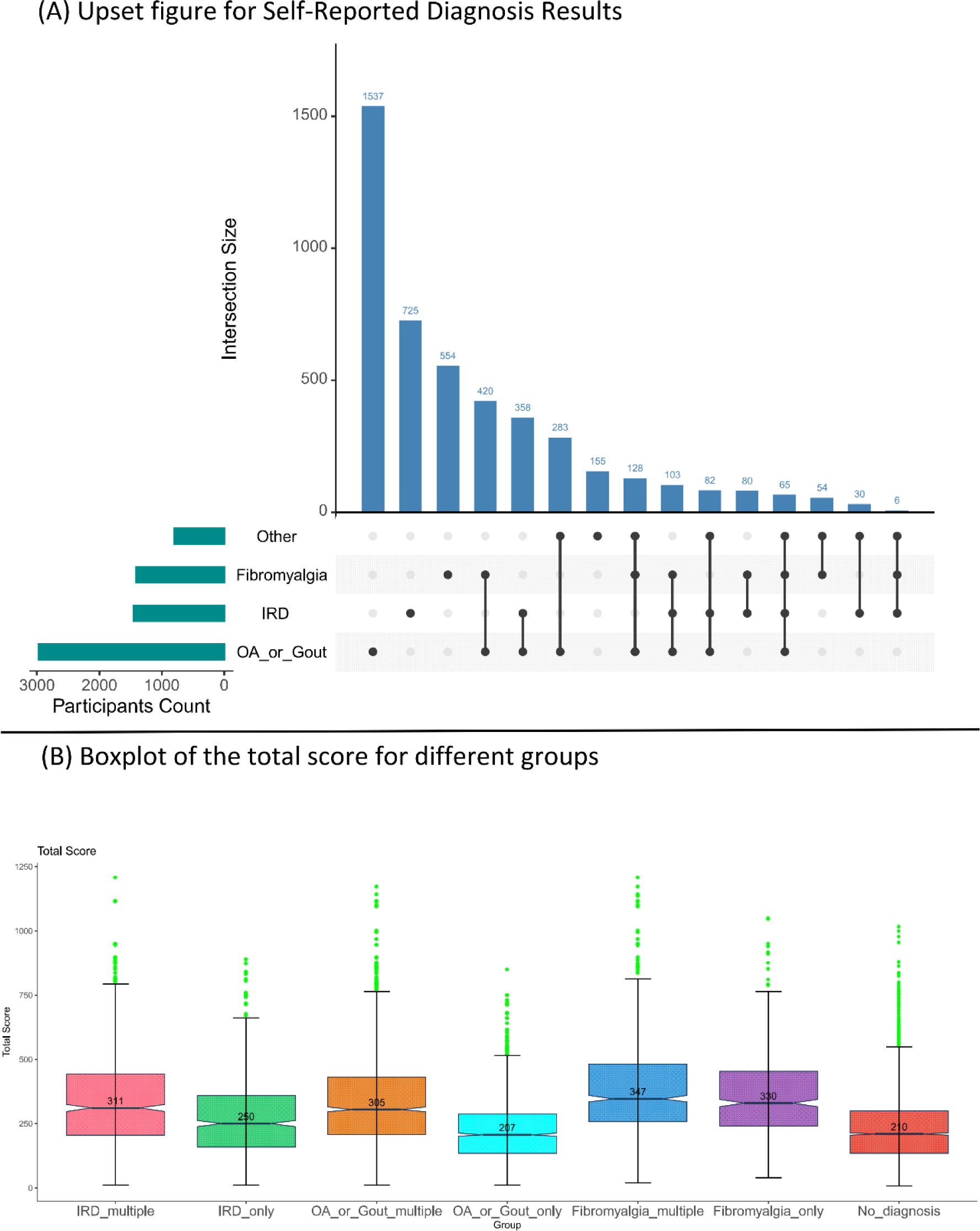
Results: 8,727 participants were included, of which 6861(79%) were female. The distribution of self-reported diagnoses is shown in Figure 1A. Variations in total scores across diagnosis groups is shown in Figure 1B. Among the participants, 4,146 (48%) did not receive a diagnosis (Table 1), 1,449 (17%) reported an IRD diagnosis, 1,410 (16%) fibromyalgia, and 2,976 (34%) OA or gout. Sex distribution differed between these four diagnostic categories: 78%, 70%, 94% and 80% respectively. 35% had a diagnosis in more than one disease category (Figure 1A). 24% of the people with OA/gout also reported fibromyalgia. For IRDs this was 17.5%.
Patients without a diagnosis had a median score of 210 (Figure 1B). Consistent with the previous study, patients with only an IRD had a significantly higher score than patients with only OA or gout (IRD only 250 versus AO/Gout only 207, P<0.001 ) 2 . However, this difference was less marked for patients with multiple diagnoses (IRD multiple 311 and OA/Gout multiple 305, P=0.57 ). This was majorly caused by fibromyalgia patients whose median score was even higher than of people with an IRD (330 and 347 for FM only and FM multiple ).
Conclusion: Our study is the first to test an online symptom checker by following real-life patients. Rheumatic? is capable of capturing different patterns between patients with and without immune-mediated rheumatic diseases by scoring IRDs higher than non-IRDs. However, the magnitude of the difference is obscured by people having multiple diagnoses in particular fibromyalgia.
REFERENCES: [1] Knitza J, et al. DOI: 10.2196/17507.
[2] Knevel R, et al. DOI: 10.3389/fmed.2022.774945.
Table 1. Patient characteristics and self-reported diagnoses.
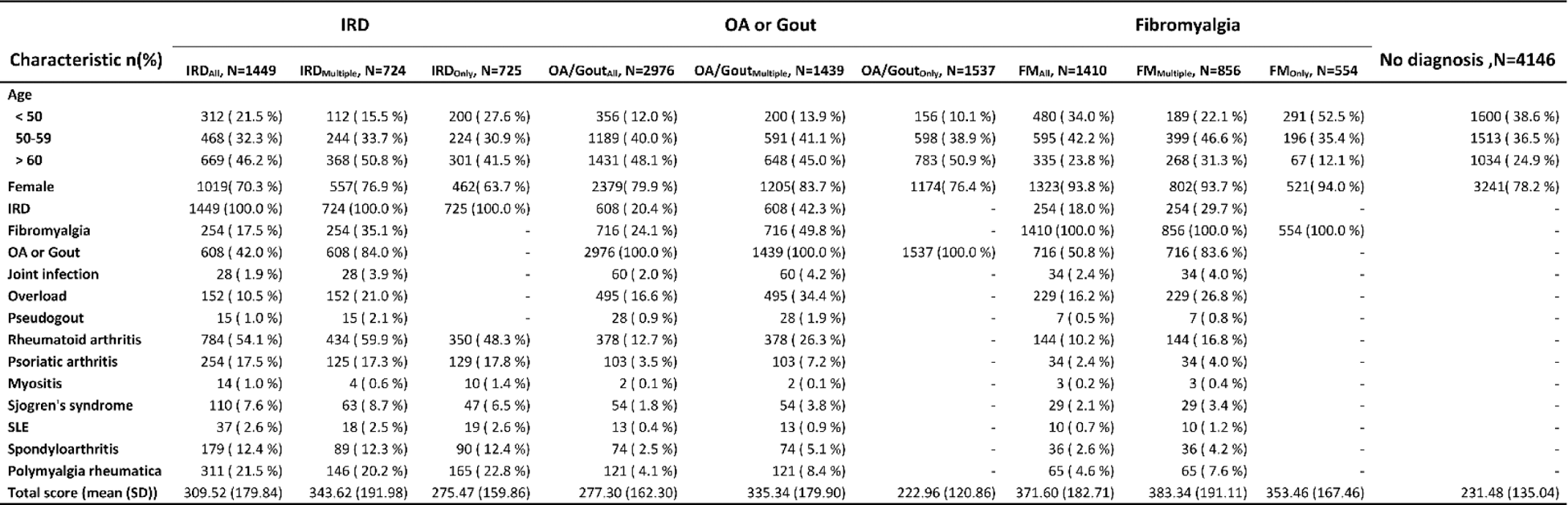
IRD = immune mediate rheumatic diseases, OA = osteoarthritis, FM = fibromyalgia, only = selection of patients with only a diagnosis in the specified category, multiple = diagnoses in multiple disease categories, all = all patients with a specified category diagnosis
Other = joint infection, pseudogout and overload for musculoskeletal system
Acknowledgements: This study was supported by Horizon-EU within the SPIDERR project (activity No.101080711), and the DigiPrevent program (funded in April 2022) from EIT Health. The project has further received funding from the Dutch Organization for Health Research and Development (ZonMw) via the Klinische Fellow No. 40-00703-97-19069, and Open Competition, No. 09120012110075. A special thank you to David Steeman for data management.
Disclosure of Interests: Ling Qin: None declared, Floor Zegers: None declared, Daniyal Selani: None declared, Erik B. van den Akker: None declared, Reinhard Bos: None declared, Saskia Le Cessie: None declared, Isabel Gehring Thermo Fisher Scientific, Herman Kasper Glas: None declared, Martina Johannesson: None declared, Johannes Knitza: None declared, Linda Mathsson-Alm Thermo Fisher Scientific, Marcel J.T. Reinders: None declared, Ewout Steyerberg: None declared, Astrid van Tubergen: None declared, Lise M. Verhoef: None declared, Barbara Axnäs ELSA Science AB, Lars Klareskog: None declared, Rachel Knevel: None declared.