

Background: Despite significant impact on cancer patient treatment and survival, usage of immune checkpoint inhibitors (ICI) carries a risk of adverse events[1,2]. Some of those ICI-related adverse events (R-irAEs) resemble rheumatological diseases, affecting around 10-15% of ICI-treated patients[3-5]. Due to increasing ICI-therapy prescription, patients with R-irAEs are increasingly referred for rheumatological care. Clinical diagnostic criteria and therapeutic intervention strategies for R-irAEs have not yet been determined, despite shared progress in management of these adverse events[2-5]. Clinical R-irAE data collection is needed to support future guideline development for this new, growing rheumatological patient population.
Objectives: To analyze clinical presentations that were referred with rheumatological symptoms after initiation of immune therapy in the past 2 years to the rheumatology outpatient clinic of AmsterdamUMC.
Methods: All initial presentations and related follow-up data and visits from January 1 st of 2022 to December 28 th of 2023 were extracted from the outpatient clinic registry and retrospectively analyzed. Evaluation included clinical presentation, medical history, physical exam findings, serological results, chosen treatments and subsequent response. This evaluation continued to last clinical follow-up before December 28 th 2023 or discharge from rheumatological care. The results were analyzed using descriptive statistics, evaluating incidence, pathological characteristics and treatment response.
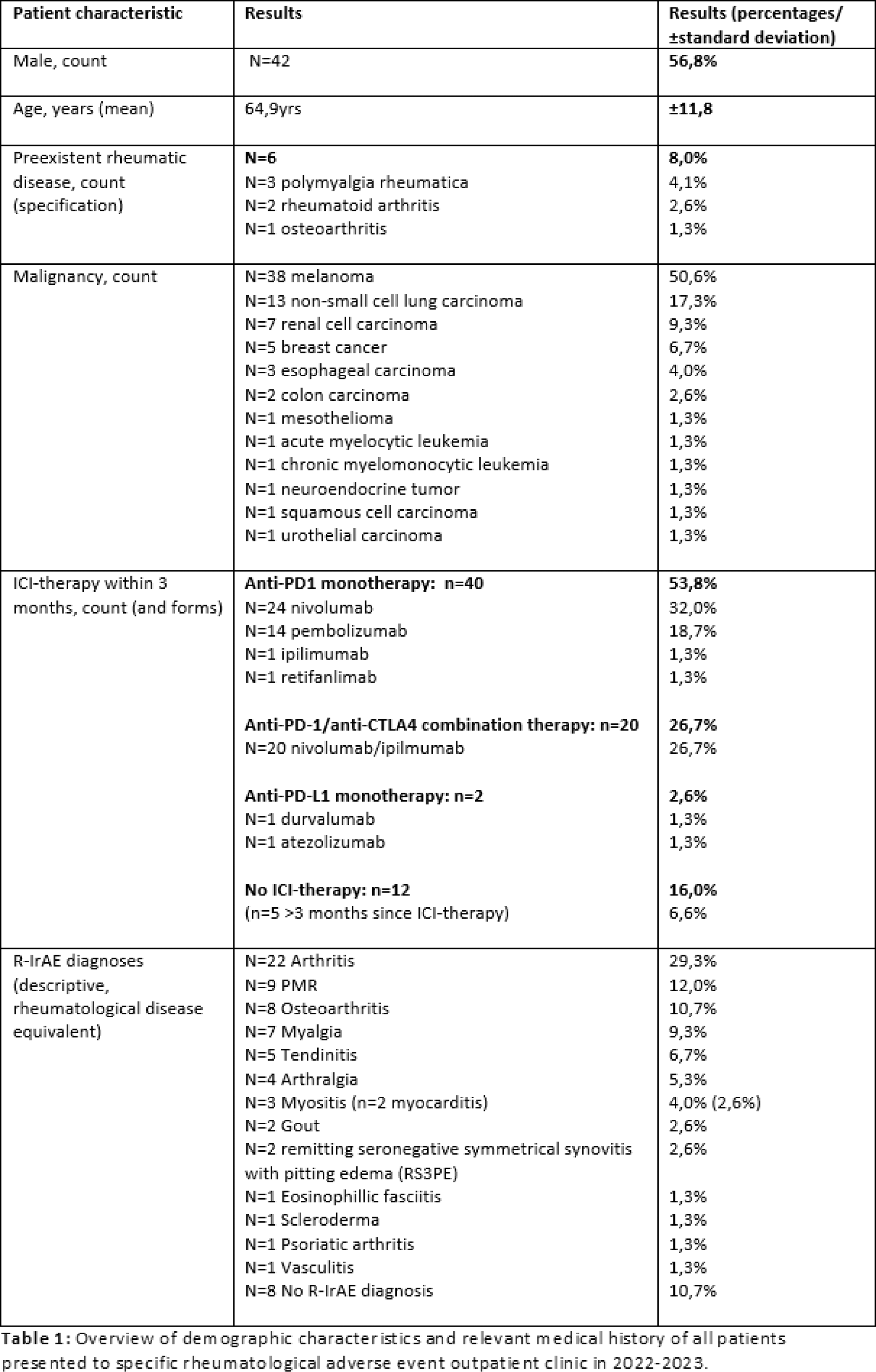
Results: A total of 74 patients were presented to the outpatient clinic over the 2-year inclusion period. Patients were 57% male and most commonly received ICI-therapy for melanoma(51%)(see Table 1 for full overview). The majority of presenting symptoms were arthralgia(91%) and/or myalgia(55%). Positive rheumatoid factor was found in 8 patients, with positive anti-CCP in 7 cases (6 RF+aCCP positive, 1 preexistent RA). Imaging was required in 6 patients with unclear symptoms, with four ultrasounds revealing tendinitis (n=2), arthritis (n=1) and no rheumatic disease (n=1), and two PET/CT scans arthritis and vasculitis (n=1 each). Overall clinical assessment yielded arthritis(29%), polymyalgia rheumatica (PMR)-like symptoms(12%), osteoarthritis-like symptoms(11%), undifferentiated myalgia(9%), other rheumatic-like diagnoses(28%) and no specific rheumatic-like disease (11%). Treatment was initiated in 49 patients, including pain medication (n=3, 4%), prednisone (n=36, 49%) and combination prednisone-methotrexate (n=10, 13%). After initial treatment, 15 patients(42%) on prednisone monotherapy required augmentation within 6 months, 9 patients(25%) receiving additional methotrexate and 6 patients(17%) additional tocilizumab respectively. At mean follow-up of 5,7 months (±5,0) 46 patients(62%) had achieved R-IrAE remission. All other patients had mostly a reported decreased disease burden at latest evaluation (34% still in rheumatological follow-up). In n=12(16%) patients, ICI therapy was discontinued of whom n=8(11%) permanently and n=4(6%) temporarily. By the end of our evaluation, n=42(57%) patients showed response to treatment with n=8(11%) showing no remaining malignancy at last evaluation. The other n=32(43%) patients had progressive disease, n=9(12%) of whom had died.
Conclusion: Based on the results of this clinical analysis, patients presented with diverse rheumatological disease-like symptomatology after initiation of immunotherapy. Clinical arthritis occurred as most common R-irAE, requiring anti-inflammatory treatment including biologicals in a small group(8%) of patients. Most patients attained remission, but initial therapy often required augmentation indicating a need for improved diagnostic and therapeutic strategies, new guidelines, all underpinned by greater understanding of pathology.
REFERENCES: [1] Abdel-Wahab, N. et al., Rheum. 2019;58(7).
[2] Haanen, J. et al., Ann Oncol. 2020;31(6).
[3] Benfaremo, D. et al., Curr Drug Saf. 2018;13(3).
[4] Ghosh, N. et al., Med Clin North Am. 2021;105(2).
[5] Kostine, M. et al., Ann Rheum Dis. 2020;80(1).
Acknowledgements: With special thanks to the departments of “Rheumatology and Clinical Immunology” and “Medical Oncology” of the Amsterdam University Medical Centers.
Disclosure of Interests: None declared.