

Background: We recently identified, through latent class analysis, a distinct phenotype of systemic lupus erythematosus (SLE) patients who were more likely to flare after anti-SARS-CoV-2 mRNA vaccination. They were younger, had higher disease activity and a higher baseline glucocorticoid (GC) dose [1].
Objectives: To study the association of treatment with disease flares after anti-SARS-CoV-2 mRNA vaccination in a larger group of patients with connective tissue disease (CTD) and the subset with SLE.
Methods: Data were abstracted from electronic medical records for a retrospective cohort study of patients with rheumatic disease aged ≥ 12-years-old, who received at least one dose of Pfizer-BioNTech or Moderna vaccine across 8 public hospitals in Singapore. CTD included SLE, systemic sclerosis, inflammatory myositis, mixed and undifferentiated CTD and Sjogren’s syndrome. Follow-up was censored at date of known flare, at the patient’s post-vaccine clinic visit when flare was recorded, or 3 months after the initial vaccine dose, whichever came first. Baseline characteristics were compared using chi-square test for categorical; and t-test and Mann-Whitney U test for continuous variables in normal and non-normal distributions respectively. Treatment as a predictor of flare post-mRNA vaccination was analysed using Cox proportional hazard regression, adjusting for baseline characteristics.
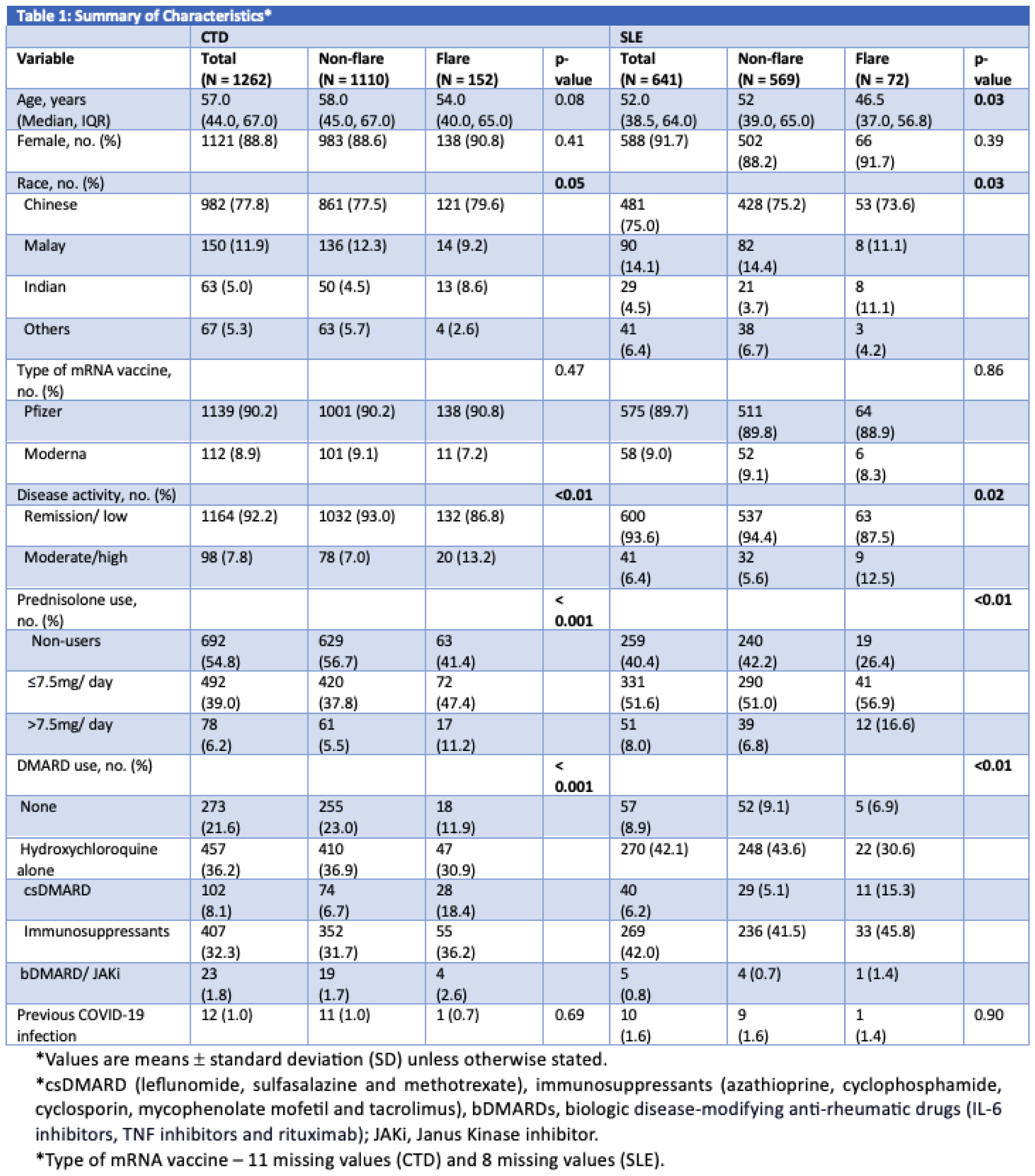
Results: 1262 CTD patients [88.8% female, 77.8% Chinese; median (IQR) age 57.0 (44.0, 67.0) years] were included. 641 had SLE (91.7% female, 75% Chinese; median (IQR) age of 52.0 (38.5, 64.0) years]. 1139 (90.2%) and 112 (8.9%) CTD, and 575 (89.7%) and 58 (9.0%) SLE patients had received Pfizer-BioNTech and Moderna vaccines, respectively. 98 (7.8%) CTD and 41 (6.4%) SLE patients had active disease, defined as moderate or high disease activity scored by the treating physician. 1.0% CTD and 1.6% SLE patients had previous COVID-19 infection. 17 CTD patients (15 SLE) had treatments paused for vaccination. 152 (12%) CTD and 72 (11.2%) SLE patients flared in the 3-month period of interest. Patients who flared had more active disease at baseline than those who did not flare in both CTD (13.2% vs 7.0%, p < 0.01) and SLE (12.5% vs 5.6%, p < 0.01) (Table 1) groups respectively.
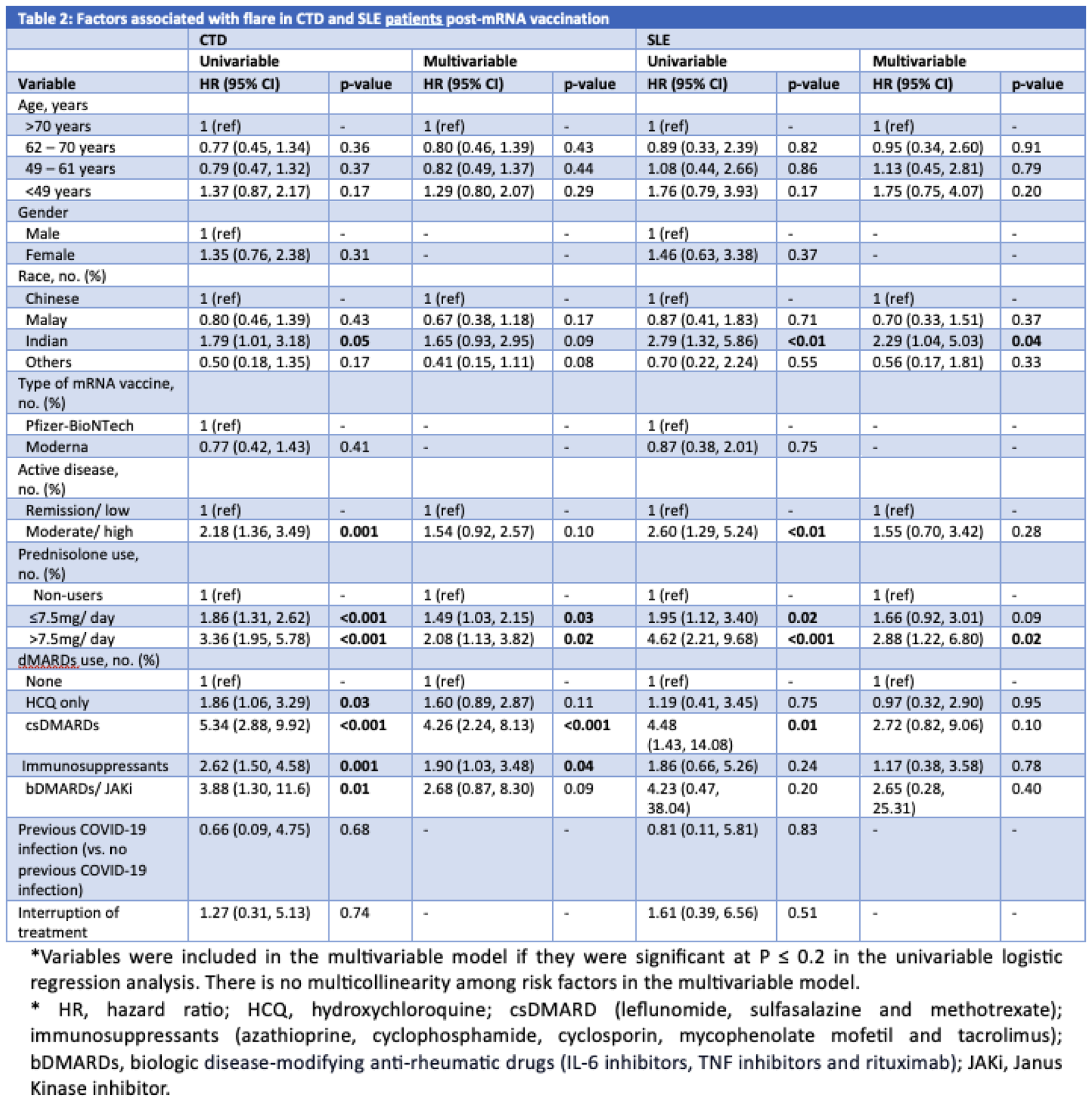
On multivariable Cox regression in CTD patients, prednisolone use ≤ 7.5mg daily [hazard ratio (HR) 1.49 (95% CI 1.03, 2.15), p = 0.03] and > 7.5mg daily [HR 2.08 (95% CI 1.13, 3.82), p = 0.02] as compared to non-use; use of conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs), (leflunomide, sulfasalazine and methotrexate) [HR 4.26 (95% CI 2.24, 8.13), p < 0.001]; and use of immunosuppressants (azathioprine, cyclophosphamide, cyclosporin, mycophenolate mofetil and tacrolimus) [HR 1.90 (95% CI 1.03, 3.48), p = 0.04] were associated with higher risk of flare, even after adjusting for baseline active disease (Table 2). On multivariable Cox regression for SLE patients, Indian race [HR 2.29 (95% CI 1.04, 5.03), p = 0.04] and prednisolone use > 7.5mg daily [HR 2.88 (95% CI 1.22, 6.80, p = 0.02] were associated with higher flare risk. Looking at types of flare in SLE patients, on univariable analysis, use of csDMARDs was associated with greater risk of flares overall [HR 4.48 (95% CI 1.43, 14.08), p = 0.01] and especially flares of inflammatory arthritis [HR 16.9 (95% CI 2.17, 132.43), p < 0.01] but not on multivariable analysis [HR 2.72 (95% CI 0.82, 9.06), p = 0.10].
Conclusion: Prednisolone > 7.5mg daily was consistently associated with an elevated flare risk in patients with CTD and the subset of SLE. GC use may be a surrogate for higher disease activity requiring more intense immunosuppression hence, heightening post-vaccination flare risk. Consideration of the dose and duration of use, frequency of prior flares, and limitation of data collection from retrospective medical records is crucial.
REFERENCES: [1] Sim TM, Lahiri M, Ma M, et al. Vaccines. 2024; 12(1):29.
Acknowledgements: NIL.
Disclosure of Interests: Preeti Dhanasekaran: None declared, Anselm Mak GSK, Margaret Ma: None declared, Yiong Huak Chan: None declared, Amelia Santosa: None declared, Peter Cheung Novartis, Novartis, Boehringer Ingelheim, Abbvie, Janssen, Warren Fong Novartis, Janssen and Janssen, GlaxoSmithKline, Diethelm Keller Siber Hegner (DKSH), Novartis, Janssen and Janssen, Abbott, Janssen and Janssen, Abbvie, Roche, Novartis, DKSH, Boehringer, Stanley Angkodjojo: None declared, Chuanhui Xu: None declared, Kok Ooi Kong: None declared, Thaschawee Arkachaisri: None declared, Kee Fong Phang: None declared, Teck Choon TAN: None declared, Melonie Sriranganathan: None declared, Sen Hee Tay: None declared, Manjari Lahiri Moderna, Astra Zeneca, Abbvie, J&J, DKSH, DKSH.