

Background: In the Sint Maartenskliniek all rheumatoid arthritis (RA), psoriatic arthritis (PsA) or axial spondyloarthritis (axSpA) patients treated with etanercept biosimilar SB4 were transitioned to etanercept bio-originator. Transition was mandatory, but a rollback option was available in case of medically objective inefficacy or side-effects. For some patients this was their second transition, as in 2016, patients participated in a non-mandatory transition from etanercept bio-originator to that biosimilar. [1] Assessment of the effects of transitioning from the bio-originator to a biosimilar has been the focus of multiple studies and is generally accepted to be effective and safe. However, evidence on multiple transitions (including back to bio-originator) is very limited, sample sizes are small and data on specifically etanercept in rheumatic diseases is limited as well.
Objectives: The aim of the current study is to investigate drug survival of etanercept bio-originator and changes in disease activity in patients who transitioned from biosimilar SB4 to bio-originator in 2022, and to assess differences between patients who previously had or had not used the bio-originator.
Methods: A retrospective cohort study of patients with a clinical diagnosis of RA, PsA or axSpA who transitioned from etanercept biosimilar to etanercept bio-originator. We identified 2 sub cohorts, cohort 1: patients who transitioned for the first time (biosimilar to bio-originator) and cohort 2: patients who transitioned before (bio-originator to biosimilar to bio-originator). Data was extracted from electronic health records. Primary outcome was drug survival of etanercept bio-originator after the transition, analysed using multivariate cox regression analyses. Data was subdivided and tested for differences between cohort 1 and 2. Secondary outcomes included change in disease activity in six months after the transition, differences in this change between cohort 1 and 2 and reasons for treatment discontinuation with etanercept bio-originator. Lastly, drug survival of the current cohort was compared to drug survival in the previous cohort of the transition from etanercept bio-originator to etanercept biosimilar in 2016 and the 2014 reference cohort that was continuously treated with bio-originator. (1)
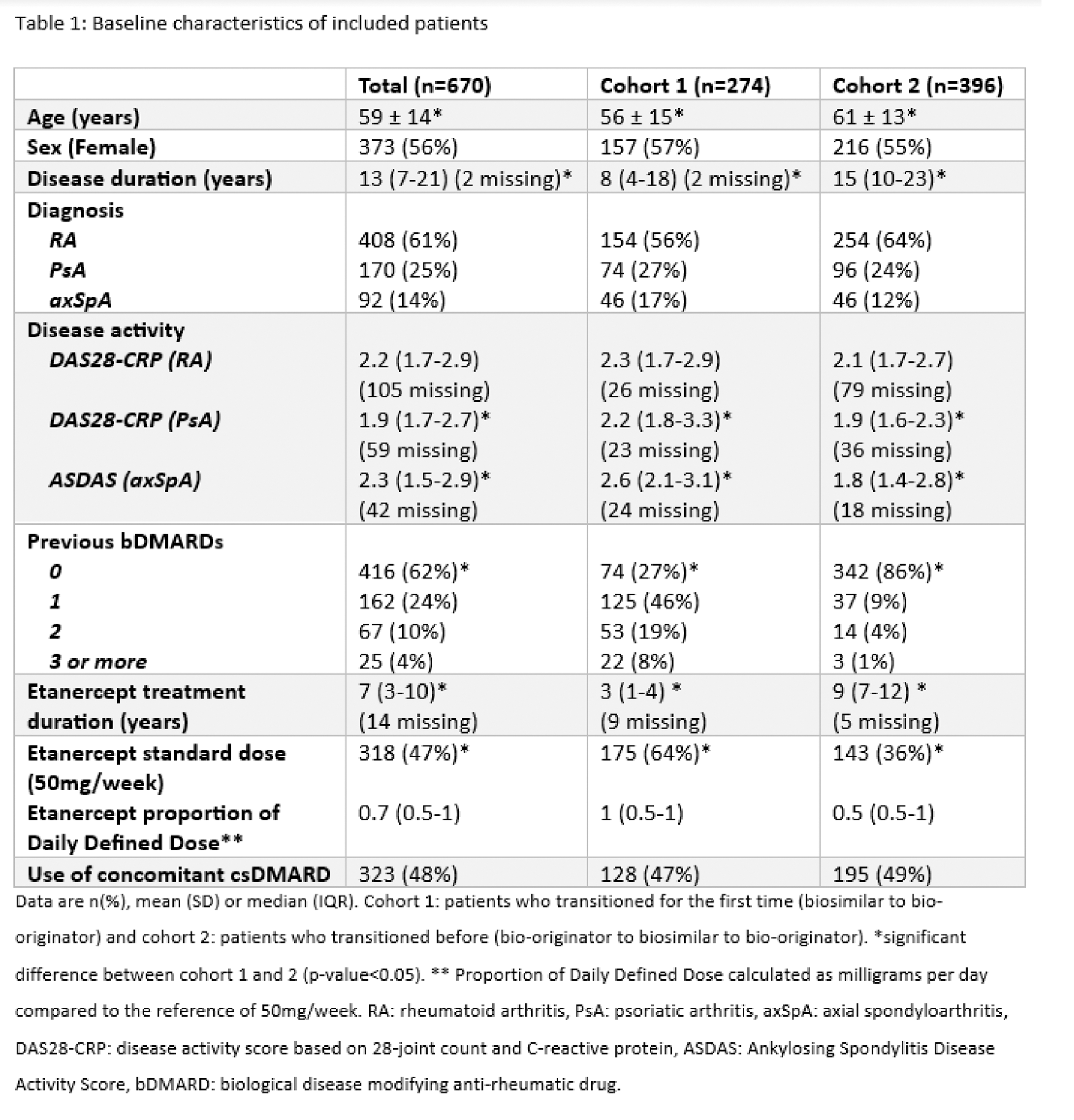
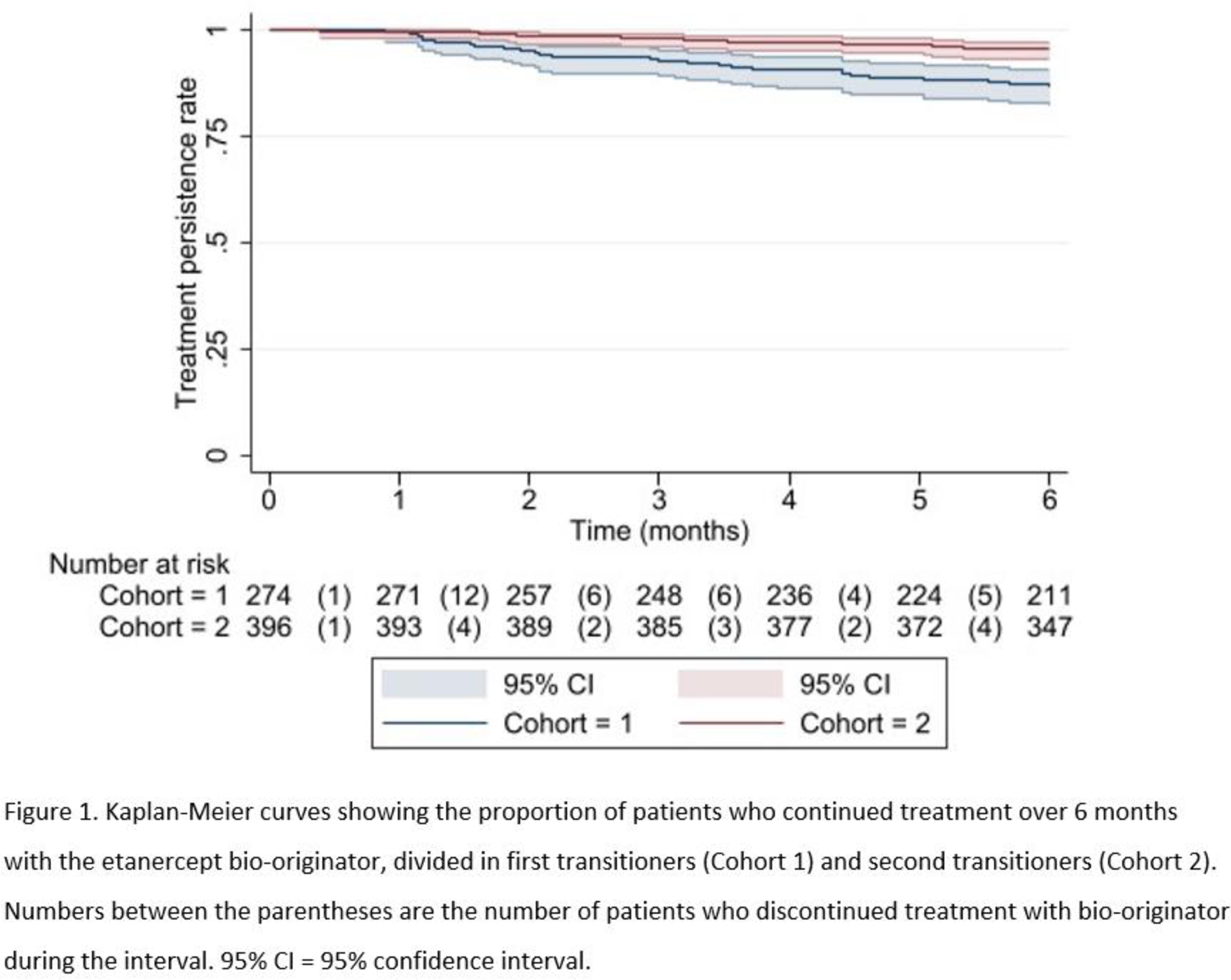
Results: 670 patients transitioned from biosimilar to bio-originator in 2022, of whom 274 belonged in cohort 1 and 396 in cohort 2. Patients in cohort 1 had significantly shorter disease duration, lower age, higher disease activity (only PsA and axSpA patients), more previous bDMARDs, shorter etanercept treatment duration and more often used etanercept in the standard dose (e.g. not tapered) compared to cohort 2 (Table 1). Overall drug survival at six months was 92% (95% CI 90-94%). For cohort 1 and 2 this was 87% (95% CI 82-90%) and 95% (95% CI 93-97%) respectively (Figure 1). Cohort 1 showed a non-significant higher risk of treatment discontinuation compared to cohort 2 (adjusted HR 1.28, [95% CI 0.57-2.86]). Reasons for discontinuation of treatment with bio-originator in Cohort 1 (n=32) and Cohort 2 (n=18) were perceived ineffectiveness (72% vs 78%) and perceived adverse events (28% vs 22%, mostly injection site reactions). Disease activity did not significantly differ between baseline and six months. The 2022 cohort showed, similar to the 2016 transition cohort, a significant higher risk of treatment discontinuation compared to the 2014 reference cohort that did not transition (HR 1.70, 95% CI 1.11 – 2.6, p-value < 0.05).
Conclusion: Overall etanercept survival rates are high and disease activity measures are on a group level not affected by the transition. No significant differences were found between patients who previously had or had not used the bio-originator. Therefore, transitioning back and forth between biosimilar and bio-originator can be used in clinical practice to achieve cost-effective care.
REFERENCES: [1] Tweehuysen L et al. Transitioning From Originator Etanercept to Biosimilar SB4: Six-Month Results From a Controlled Cohort Study. Arthritis and Rheumatology. 2018;70(9):1408-18.
Acknowledgements: NIL.
Disclosure of Interests: None declared.