

Background: During the COVID-19 pandemic, telemedicine was widely adopted as an approach to care for people with rheumatic diseases. Data on whether telemedicine is as good as in-person care in terms of patient satisfaction and effectiveness of care in rheumatology is limited.
Objectives: We aimed to determine whether telemedicine visits were noninferior to in-person visits for patient satisfaction and other measures of care effectiveness.
Methods: We conducted a parallel group, randomized, single-blind, noninferiority trial in rheumatology clinics at two academic medical centers in the United States during August 2021 to December 2022. Eligible patients were age ≥ 18 years with at least one rheumatic disease, who had ≥ 2 clinic visits in the previous 18 months (telemedicine or in-person), one of which was an in-person visit. The primary outcome was post-visit satisfaction rate (9 or 10 on a 0 – 10 satisfaction scale, higher values represent higher satisfaction). We also collected secondary outcome data on preference for the next visit type, self-efficacy for managing medications, and medication adherence. Preference for the next visit type was defined as: same as the group allocation, different than the group allocation or no preference. Exploratory outcomes included healthcare utilization after the visit (e.g., emergency room visits or hospitalizations), time missed from work and expenses incurred related to attending the visit, and appropriate laboratory monitoring based on medication use. Primary outcome was determined by assessing if the satisfaction rate with telemedicine versus in-person visits was noninferior, using a noninferiority margin of 10%. We performed modified intent-to-treat (mITT), and per protocol (PP) analyses to account for those that crossed over.
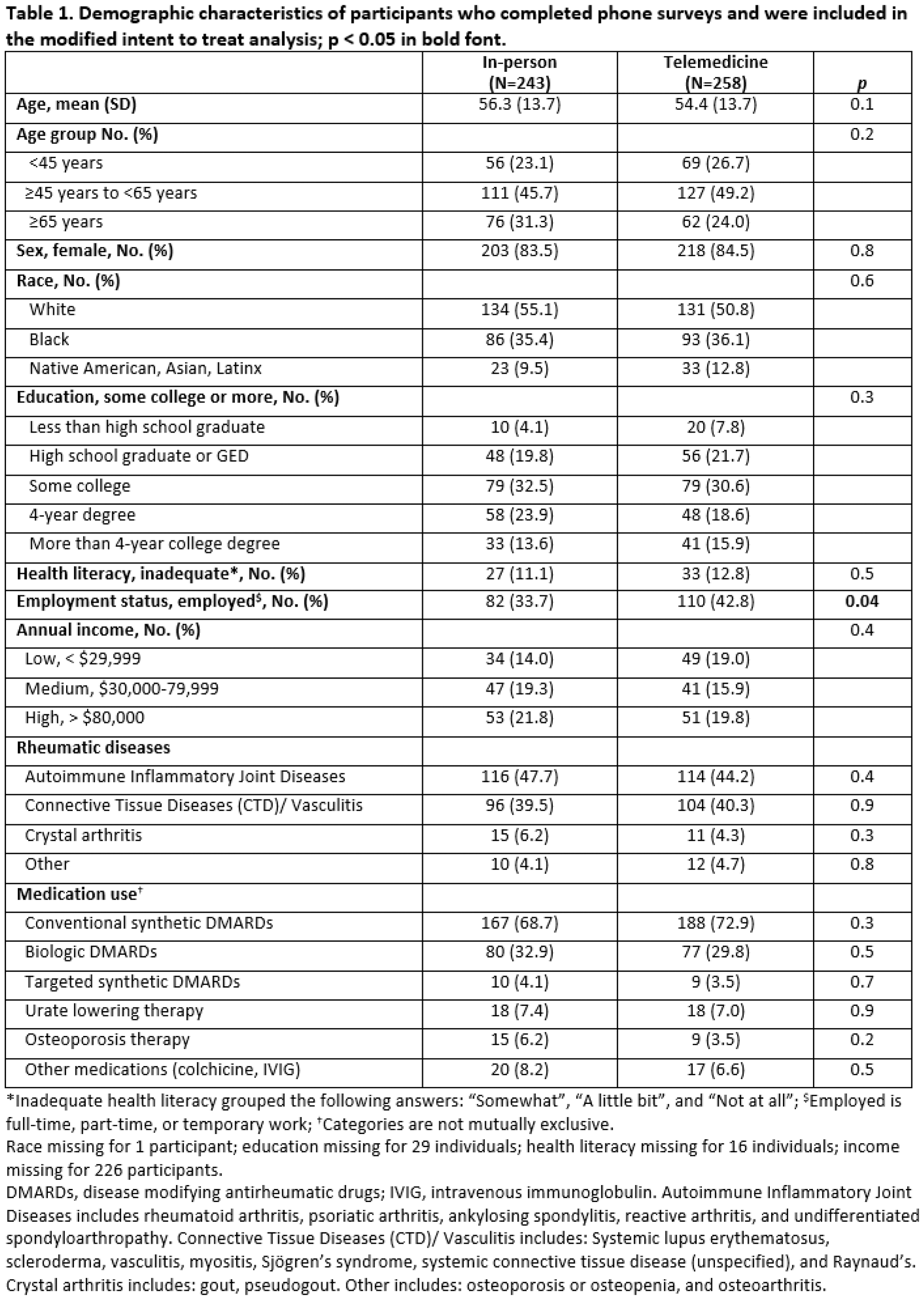
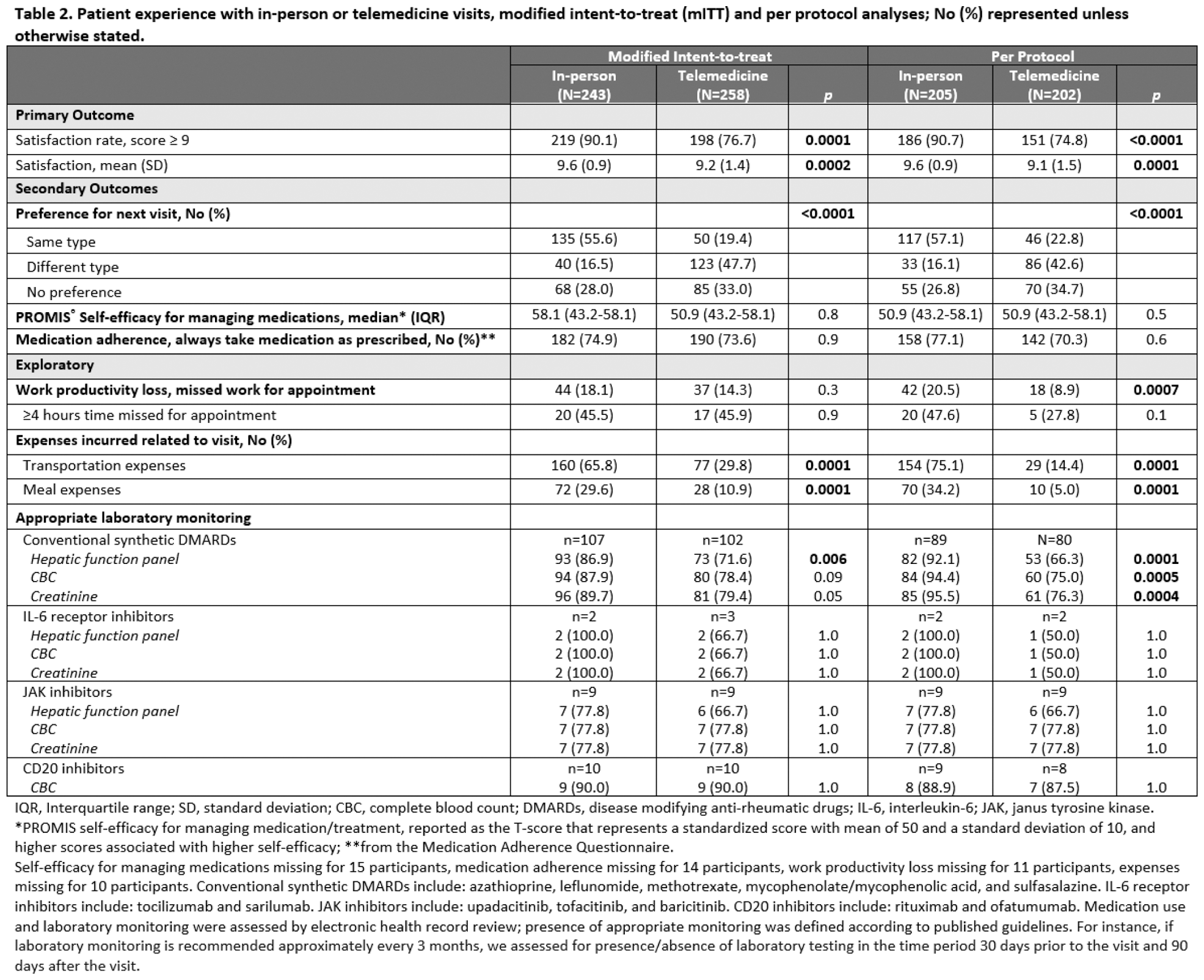
Results: Out of 652 randomized participants, 501, 35.7% Black, 84.0% women, mean age 55.3 years completed surveys. In the mITT analysis, we were unable to reject the noninferiority hypothesis and also found that telemedicine visits were inferior to in-person visits for the proportion of people that were highly satisfied, 76.7% in the telemedicine group vs. 90.1% in the in-person group, difference 13.4% (95% CI, 7.0% to 19.8%). By arm, 38 participants crossed over in the in-person group and 46 in the telemedicine group (p = 0.5). In the PP analysis, the satisfaction rate for telemedicine visits was also inferior to in-person visits, difference 16.0% (95% CI, 8.8% to 23.3%). The proportion of participants who indicated they preferred the same type of visit for their next visit compared to a different visit type or no preference was significantly greater for those who had in-person visits (mITT analysis, 55.6% vs 19.4% in-person vs telemedicine, p < 0.0001). In both the mITT and PP analyses, most participants preferred an in-person visit for their next visit. There were no clinically or statistically significant differences between groups in self-efficacy for managing medications or medication adherence in either the mITT or PP analyses. In PP analyses, significantly more individuals in the in-person group vs. the telemedicine group had appropriate laboratory monitoring for conventional synthetic DMARDs, and more had reported expenses related to meals and transportation. There were no significant differences in healthcare utilization between groups.
Conclusion: Among a large group of geographically, racially and ethnically diverse established rheumatology patients, although a majority of patients were satisfied with telemedicine visits, high patient satisfaction with rheumatology clinic visits and appropriate laboratory monitoring was lower for telemedicine than for in-person visits. Most participants preferred an in-person visit as their next visit type. Future studies should focus on methods to improve quality of care delivered by telemedicine.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Lesley Jackson: None declared, Justin Leach: None declared, Jinoos Yazdany Astra Zeneca, Aurinia, Gilead, Pfizer, Kenneth Saag Abbvie, Amgen, Arthrosi, Atom Bioscience, Bayer, CSL Behring, Daiichi Sankyo, Gilead, Horizon, Inflazome, LG Pharma, Mallinkrodt, Radius, Roche/Genentech, SOBI, Takeda, Allena, Amgen, Arthrosi, Dyve, Horizon, LG Chem, Radius, Shanton, SOBI, Takeda, Ultragenex, Jeffrey R Curtis AbbVie, Amgen, Bristol-Myers Squibb, CorEvitas, Eli Lilly and Company, Janssen, Myriad, Novartis, Pfizer, Sanofi, UCB, AbbVie, Amgen, Bristol-Myers Squibb, CorEvitas, Eli Lilly and Company, Janssen, Myriad, Novartis, Pfizer, Sanofi, UCB, Diana Paez: None declared, Sarah Goglin: None declared, Mary Margaretten: None declared, David Chae: None declared, Gary Cutter Alexion, Antisense Therapeutics, Avotres, Biogen, Clene Nanomedicine, Clinical Trial Solutions LLC, Entelexo Biotherapeutics, Genentech, Genzyme, GW Pharmaceuticals, Hoya Corporation, Immunic, Immunosis Pty Ltd, Klein-Buendel Incorporated, Merck/Serono, Novartis, Perception Neurosciences, Protalix Biotherapeutics, Regeneron, Roche, SAB Biotherapeutics, Maria Danila RheumNow, UCB, Horizon, Pfizer.