

Background: Tuberculosis (TB) remains an endemic disease and a high latent tuberculosis infection (LTBI) prevalence in patients with rheumatic diseases was demonstrated in Hong Kong [1].
Initial trials and registries have shown that biologic and targeted synthetic disease-modifying anti-rheumatic drugs (b/tsDMARDs) are associated with a heightened risk of TB or LTBI reactivation.
Biologic agents directed against inflammatory pathways other than TNF-α, including IL-12, IL-17 and IL-23, work with mechanisms scarcely or not involved in the immune response against TB. However, TB risk data of these agents outside clinical trial setting are limited.
Objectives: We aimed to investigate the real-world risk and predictors of TB in rheumatic patients on non-anti-TNF b/tsDMARDs in a population with high incidence of TB.
Methods: We reviewed all adult (age ≧18) patients with rheumatic disease (rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, psoriatic arthritis, scleroderma, dermatomyositis, polymyositis, systemic vasculitis, adult onset Still’s disease, Behcet’s disease and mixed connective tissue disease) who received non-anti-TNF b/tsDMARDs at Prince of Wales Hospital, Hong Kong between 1st January 2009 to 31st December 2019. After excluding patients who had received anti-TNF bDMARDs within 1 year, these patients (non-anti-TNF b/tsDMARD group) were matched to patients who received conventional synthetic (cs)DMARDs only (non-biologic group) with same rheumatic disease in the ratio of 1:1.
The TB incidence rate of each group will be determined by dividing the new TB cases by the total patient-year, and comparisons of incidence rate ratios will be made using a Poisson model. Potential risk factors for TB disease will be determined using univariate and multivariate Poisson regression.
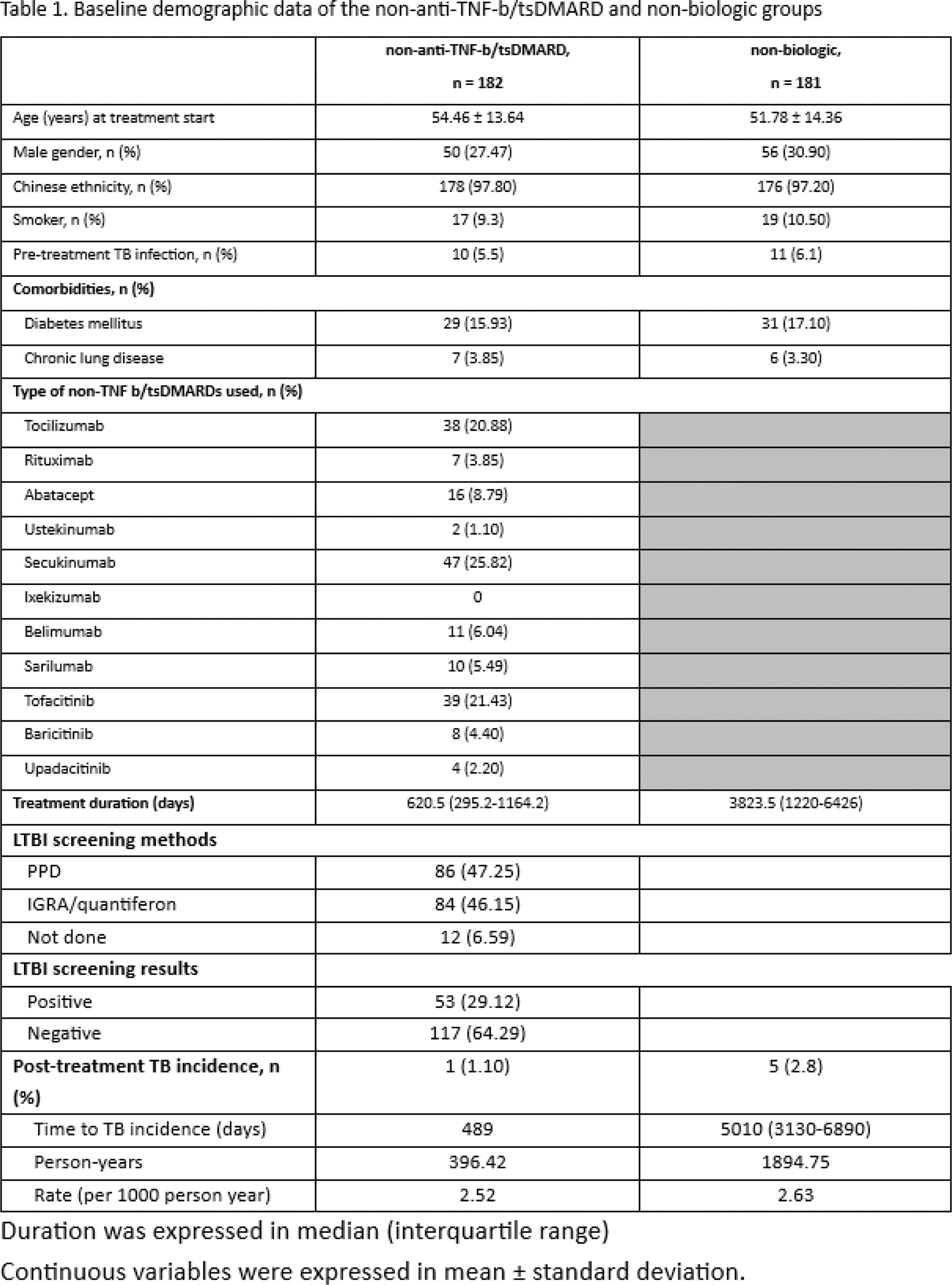
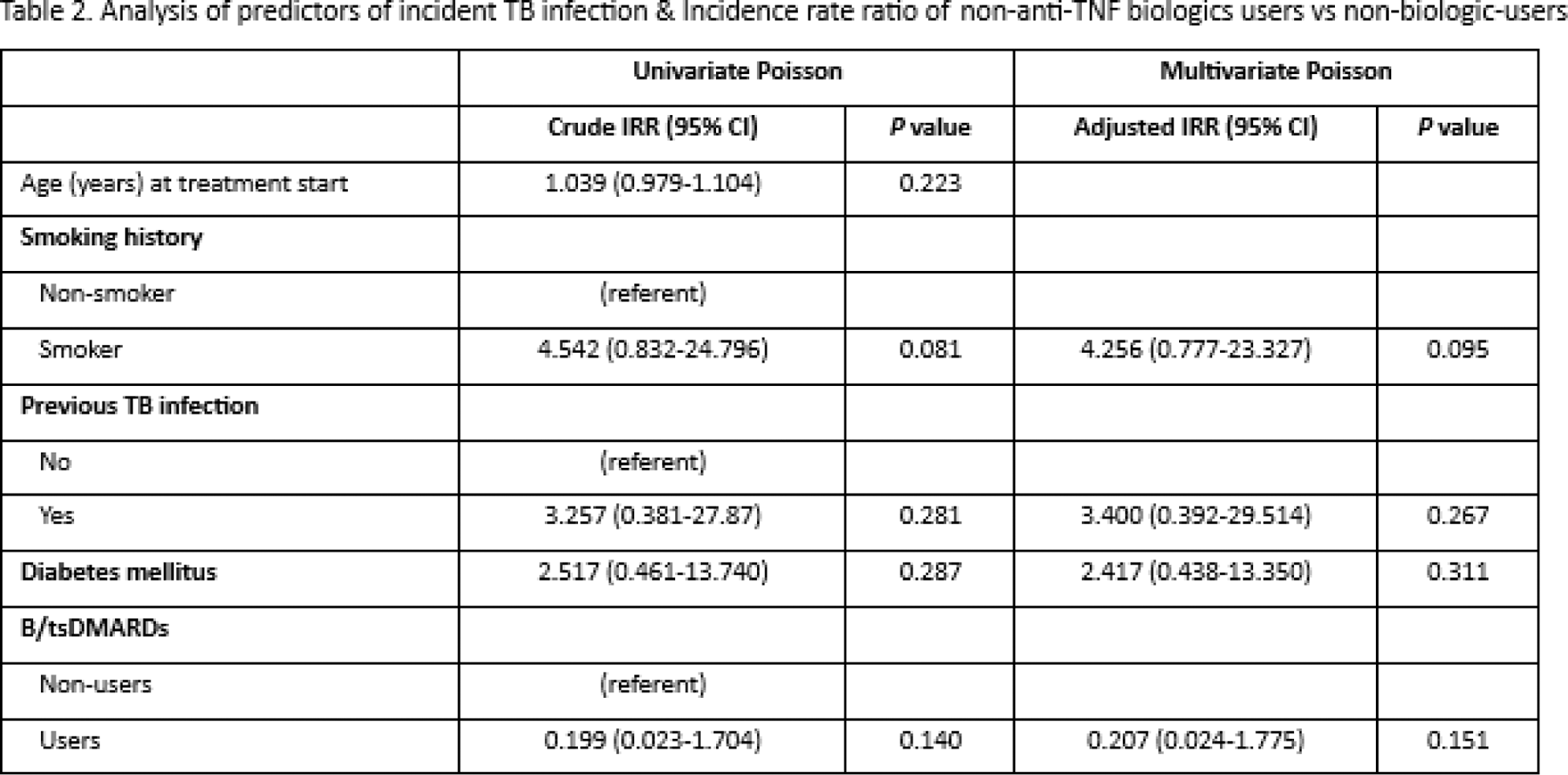
Results: In this retrospective cohort study, a total of 363 patients with rheumatological disease were included (age 53.1 ± 14.4, 106 (29.3%) male). The baseline characteristics of the 182 patients on non-anti-TNF-b/tsDMARDs and 181 patients on csDMARDs are shown in Table 1. A similar proportion of patients had past-TB infection prior to treatment in both groups (non-anti-TNF-b/tsDMARD: 5.5%, non-biologic: 6.1%). LTBI tests (47.3% by tuberculin skin test and 46.2% by interferon gamma release assay [IGRA]) were positive in 29.1% of patients before b/tsDMARDs. After a median follow-up of 3.5 years (IQR: 1.4-8.8 years), 6 post-treatment incident TB were identified, with 1 case from the non-anit-TNF-b/csDMARD group and 5 cases from the non-biologic group. The total incidence rate was 2.61 per 1000 person-years, and the rate was similar in the non-anti-TNF-b/tsDMARD group (2.52 per 1000 person-years) and the non-biologic group (2.63 per 1000 person-years). The incidence rate ratio (IRR) associated with users of non-anti-TNF-b/tsDMARD versus non-biologic-users was 0.207 (95% CI, 0.024-1.775) with p=0.151, based on a multivariate Poisson distribution. The only one patient in the non-anti-TNF-b/tsDMARD group developed incident pulmonary TB with pleural effusion 489 days after tofacitinib treatment. The pre-treatment LTBI test by IGRA was positive and the patient was given 9 months of isoniazid. Multivariate Poisson regression analysis did not reveal any significant predictors of incident TB infection in the whole cohort (Table 2).
Conclusion: This study provides one of the first real-world data which suggests the risk of TB infection for patients taking non-anti-TNF b/tsDMARDs does not exceed that of csDMARDs even in an endemic area. This could be accounted for by the successful LTBI screening and treatment strategy currently in use or the lack of influence on anti-TB immunity by these agents. Whether it is necessary and cost-effective to perform routine LTBI screening before non-anti-TNF b/tsDMARD treatment remains to be answered.
REFERENCES: [1] So H, Yuen CS, Yip RM. Comparison of a commercial interferon-gamma release assay and tuberculin skin test for the detection of latent tuberculosis infection in Hong Kong arthritis patients who are candidates for biologic agents. Hong Kong Med J. 2017;23(3):246-50.
Acknowledgements: NIL.
Disclosure of Interests: None declared.