

Background: Musculoskeletal (MSK) symptoms are the leading contributor to various diseases and disability, affecting around 1.7 billion people globally. Early disease stratification is important to ensure appropriate and timely care (most suited healthcare provider and best treatment choice). Currently the patient journey to diagnosis and effective treatment is long and inefficient, resulting in persistent disease burden and economic loss. Suboptimal care is due to inadequate access to healthcare, insufficiently understood disease etiology, similar symptoms of different diseases, lack of discriminatory tests and a trial-and-error approach in treatment.
Objectives: SPIDeRR aims to disentangle the real-life complexity of early diagnosis of musculoskeletal problems in general, and rheumatic diseases in particular, by considering the complete picture of factors influencing patients’ symptoms. It is a unique and first-in-kind consortium of 18 partners that connects all stakeholders including academic institutions, foundations/networks (o.a. patient organization) and biotech companies within Europe.
Methods: SPIDeRR’s approach goes well beyond the state-of-the-art by:
developing novel high throughput methods to analyze high dimensional multimodal clinical and biologic data from various European healthcare systems to capture and predict patient trajectory towards Rheumatic disease diagnosis.
generating supervised machine learning models that facilitate patient stratification with/without rheumatic diseases underlying similar symptoms through integration of all relevant data dimensions (symptoms, comorbidities, social circumstances, electronic health records (EHR), biomarkers and genetics) from different healthcare levels (primary and secondary care as well as patients independently seeking advice online).
expediting diagnosis through integration of patients’ clinical and biological data and enabling physicians to promptly choose a personalized therapy, which will take into account specific molecular pathways and short-and long-term effects, including risks for co-morbidities.
generating a real time and reliable pan-European rheumatic patient journey map in both qualitative and quantitative manner that will develop new pipelines for translational data science and yield opportunities for novel therapies and treatment strategies.
carry out usability and acceptability studies to ensure alignment of the developed tools to end users in their context, and in turn, improve adoption and uptake of SPIDeRR tools.
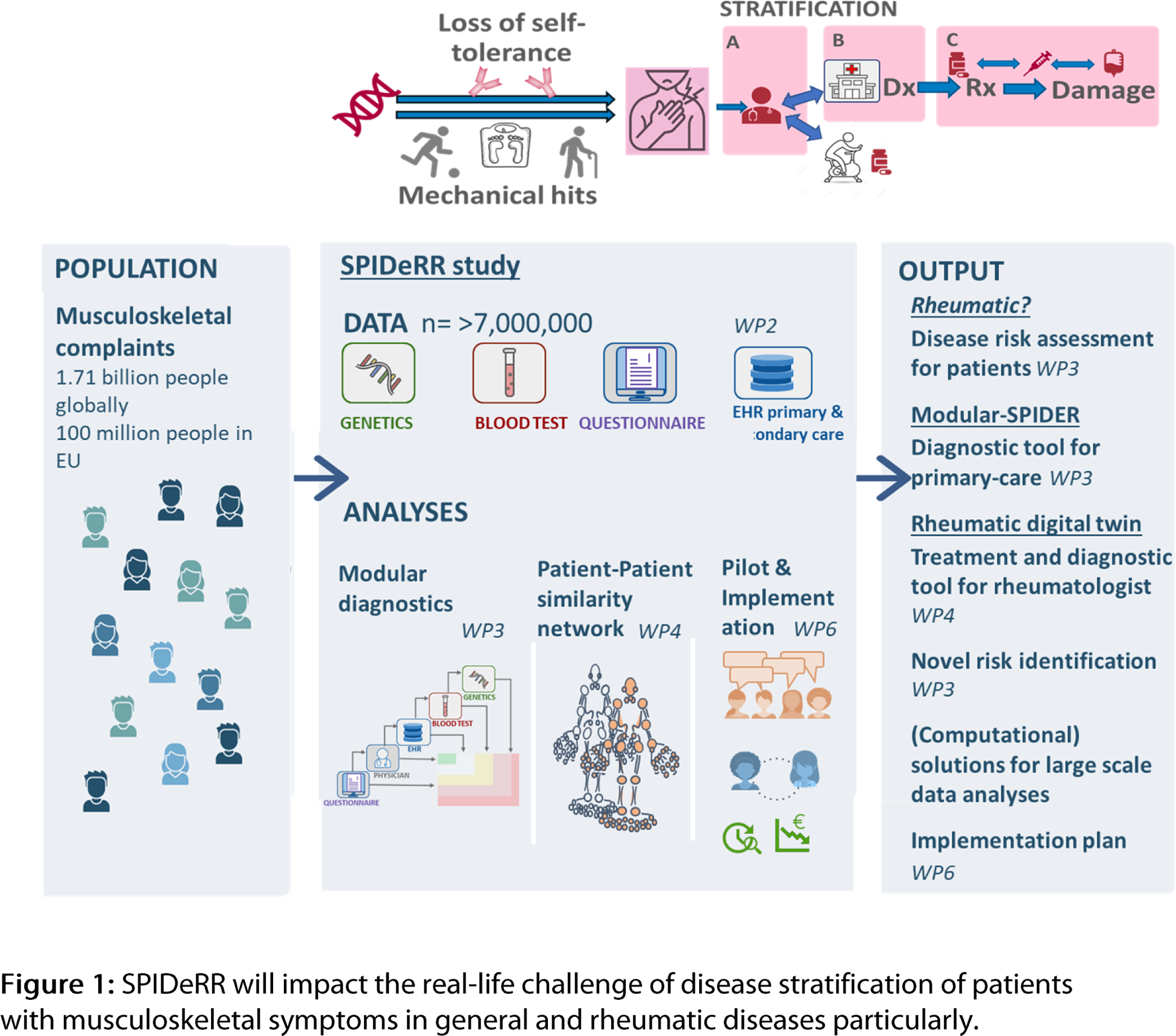
Results: The consortium aims to deliver validated, evidence-based and data-driven diagnostic and treatment support tools for all stakeholders in the healthcare value chain (Figure 1). Specifically, SPIDERR will deliver three clinical models tailored to European countries 1) Rheumatic? A user-friendly online symptom checker for patients with MSK complaints, 2) Modular-SPIDeRR. A decision support tool for primary care providers that guides for diagnostic tests and secondary care referrals, 3) Rheumatic Digital Twin . A patient-patient similarity network to optimize diagnostic groups in rheumatology and support treatment decisions. To achieve this, we will additionally deliver solutions for data integration and shared analyses though a general data protection regulation (GDPR) compliant digital research environment and federated learning pipelines. Finally, we will test the acceptability and feasibility of the models through stakeholders’ studies and provide an implementation scene tailored to current healthcare in Europe.
Conclusion: SPIDeRR, promises to achieve high impact through transformation of the patient pathway, specifically at the rheumatology level, from the earliest stages of disease development — stemming from genetic predisposition combined with environmental risk factors — towards first symptoms, the onset of chronic disease and identification of the optimal personalized treatment.
REFERENCES: NIL.
Acknowledgements: SPIDeRR is funded by the European Union under grant agreement no. 101080711.
Disclosure of Interests: None declared.