

Background: Accurate and rapid diagnosis of rheumatic joint diseases is essential for further treatment decision. Early treatment initiation of different rheumatic diseases slows the progression and positively influence their courses [1-3]. Different rheumatic diseases affecting the hands present characteristic patterns and features in fluorescence optical imaging (FOI) [4,5].
Objectives: We tested an atlas of image features in FOI for their ability to differentiate various rheumatic joint diseases such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), connective tissue disease (CTD) and osteoarthritis (OA), with the aim to simplify the early diagnostic process for rheumatic diseases affecting the hands by the identification of specific FOI criteria for each disease.
Methods: FOI images from patients with RA, PsA, CTD and OA were analyzed by two readers blinded for diagnosis and calibrated against each other, using the prima vista mode (PVM) and the 5-phase model. Twenty-six different features were pre-defined and all images were scored according to these features. The feature frequency in each patient and phase (PVM, 5-phase) was counted and statistically analysed using a contingency table and common formulae: χ 2 , Diagnostic Odds ratio (DOR), true positive rate (TPR), true negative rate (TNR), positive predictive value (PPV), and negative predictive value (NPV). In a first step, OA as a non-inflammatory disease was compared with the other diseases (RA, PsA, CTD), while these diseases were distinguished from each other in a second step.
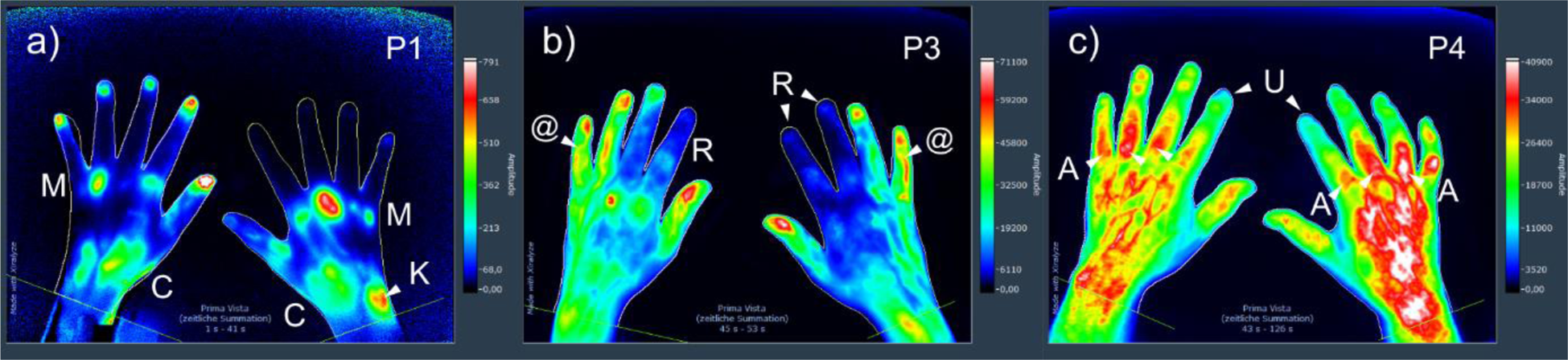
Results: A total of 374 patients (N=115 RA patients; mean age 54.4; SD 12.6), PsA (N=59; 50.0; 11.4), CTD (N=111; 52.2; 14.9) and OA (N=89; 62.1; 7.4) were included in the analysis. In a first step, OA is best distinguished and excluded from the other diseases by the means of the five features M, K, r, R and U (Table 1). In a second step, the other three diseases can be differentiated from each other including 5 features for PsA and CTD vs. RA, 10 features for RA and CTD vs. PsA, and 4 features for RA and PsA vs. CTD) (Table 2). Examples of different features in FOI are given in the Figure 1.
Conclusion: FOI feature analysis helps to differentiate typical rheumatic diseases affecting the hands from each other, which could both speed up and improve the diagnostic process, especially in the early arthritis clinic.
REFERENCES: [1] Smolen JS, et al. Ann Rheum Dis 2023;82:3–18.
[2] Smolen JS, et al. Ann Rheum Dis. 2018 Jan;77(1):3-17.
[3] Md Yusof MY, et al. Rheumatol Adv Pract. 2022;6(1):rkab106.
[4] Gedat E, et al. Diagnostics (Basel). 2022 Jul 22;12(8):1775.
[5] Rothe F, et al. Front Med (Lausanne). 2023 Aug 21;10:1228833.
OTHER – OA
| OTHER – OA | |||||||
|---|---|---|---|---|---|---|---|
| FEATURE | PHASE | χ2 | DOR | TPR | TNR | PPV | NPV |
| R | 3 | 12.0 | 8.24 | 0.08 | 0.99 | 0.97 | 0.22 |
| M | 1 | 19.1 | 3.50 | 0.21 | 0.93 | 0.92 | 0.23 |
| K | 1 | 3.6 | 2.34 | 0.07 | 0.97 | 0.90 | 0.21 |
| r | 1 | 8.7 | 2.82 | 0.13 | 0.95 | 0.91 | 0.22 |
| R | 2 | 9.35 | 3.03 | 0.12 | 0.96 | 0.91 | 0.22 |
| r | 2 | 8.65 | 4.15 | 0.09 | 0.98 | 0.94 | 0.22 |
| U | 4 | 8.10 | 2.49 | 0.14 | 0.94 | 0.90 | 0.22 |
| M | 4 | 8.16 | 2.59 | 0.13 | 0.94 | 0.90 | 0.22 |
| U | 5 | 7.75 | 2.94 | 0.11 | 0.96 | 0.91 | 0.22 |
PsA and CTD – RA, RA and CTD – PsA, PsA and CTD – RA
| PsA and CTD – RA | |||||||
|---|---|---|---|---|---|---|---|
| FEATURE | PHASE | χ2 | DOR | TPR | TNR | PPV | NPV |
| A | 3 | 14.0 | 16.8 | 0.07 | 1.00 | 0.97 | 0.35 |
| W | 1 | 16.1 | 2.38 | 0.27 | 0.87 | 0.80 | 0.37 |
| U | 2 | 5.41 | 2.46 | 0.08 | 0.97 | 0.82 | 0.35 |
| B | 3 | 9.79 | 2.33 | 0.16 | 0.92 | 0.81 | 0.36 |
| T | 3 | 8.31 | 2.44 | 0.13 | 0.94 | 0.82 | 0.35 |
| RA and CTD – PsA | |||||||
| D | 1 | 7.22 | 9.61 | 0.04 | 1.00 | 0.95 | 0.32 |
| P | 1 | 17.5 | 5.85 | 0.12 | 0.98 | 0.92 | 0.34 |
| D | 2 | 7.58 | 2.69 | 0.10 | 0.96 | 0.84 | 0.33 |
| r | 2 | 4.09 | 1.94 | 0.10 | 0.95 | 0.80 | 0.33 |
| B | 3 | 19.4 | 3.95 | 0.17 | 0.95 | 0.88 | 0.35 |
| X | 3 | 10.1 | 2.27 | 0.18 | 0.91 | 0.81 | 0.34 |
| A | 3 | 3.96 | 2.59 | 0.06 | 0.98 | 0.84 | 0.32 |
| O | 3 | 12.4 | 2.21 | 0.24 | 0.87 | 0.81 | 0.35 |
| C | 3 | 15.6 | 2.42 | 0.26 | 0.87 | 0.82 | 0.35 |
| d | 3 | 19.3 | 2.75 | 0.26 | 0.89 | 0.83 | 0.36 |
| U | 4 | 10.3 | 2.41 | 0.17 | 0.92 | 0.82 | 0.34 |
| U | 5 | 7.55 | 2.34 | 0.13 | 0.94 | 0.82 | 0.33 |
| RA and PsA – CTD | |||||||
| K | 1 | 7.56 | 2.71 | 0.09 | 0.96 | 0.83 | 0.36 |
| M | 4 | 10.8 | 2.42 | 0.16 | 0.93 | 0.80 | 0.37 |
| @ | 5 | 17.5 | 2.72 | 0.22 | 0.90 | 0.81 | 0.38 |
| M | 5 | 9.61 | 2.53 | 0.13 | 0.94 | 0.81 | 0.37 |
Legend: χ2, DOR: Diagnostic Odds Ratio, TPR: True Positive Rate, TNR: True negative rate, PPV: Positive predictive value, NPV: Negative predictive value
a) Patient with RA showing features M and K in phase 1, b) Patient with CTD (systemic lupus erythematosus, SLE) showing features R, @ and other features in phase 3, and c) features U and A among other features in a patient with CTD (systemic sclerosis, SSc).
Acknowledgements: NIL.
Disclosure of Interests: Nele Stumper: None declared, Jörn Berger Joern Berger, PhD, is employed by Xiralite GmbH., Egbert Gedat Egbert Gedat, PhD, is employed by Xiralite GmbH., Paula Hoff: None declared, Gabriela Schmittat: None declared, Gerd R. Burmester: None declared, Gerhard Krönke: None declared, Marina Backhaus: None declared, Ida K. Haugen: None declared, Sarah Ohrndorf Novartis; Janssen; Mylan, Speakers’ honoraria or travel expense reimbursements by:
• AbbVie, Amgen, BMS, Galapagos, Janssen, Mylan, Novartis, UCB, Novartis; GSK; AbbVie.