

Background: Nintedanib (NINTE) has been approved for the treatment of idiopathic pulmonary fibrosis (IPF), fibrosing intersticial lung disease (ILD) associated with autoimmune disease (AD-ILD), and progressive fibrosing ILDs of any etiology. In the nintedanib trials, its efficacy was consistent across all these subgroups.
Objectives: The goals of our research were to compare the efficacy and safety of NINTE between patients with AD-ILD and patients with fibrosing lung involvement without AD, in routine clinical practice.
Methods: We performed a cross sectional retrospective, multicenter, case-control study. We included a total of 108 patients diagnosed with pulmonary fibrosis treated with NINTE. 67 subjects were AD-ILD cases and 41 were non-AD-ILD controls. Continuous variables were compared by Student’s test or Mann Whitney’s T test, and categorical variables by Chi 2 test or Fisher’s exact test. The p value <0.05 was significant.
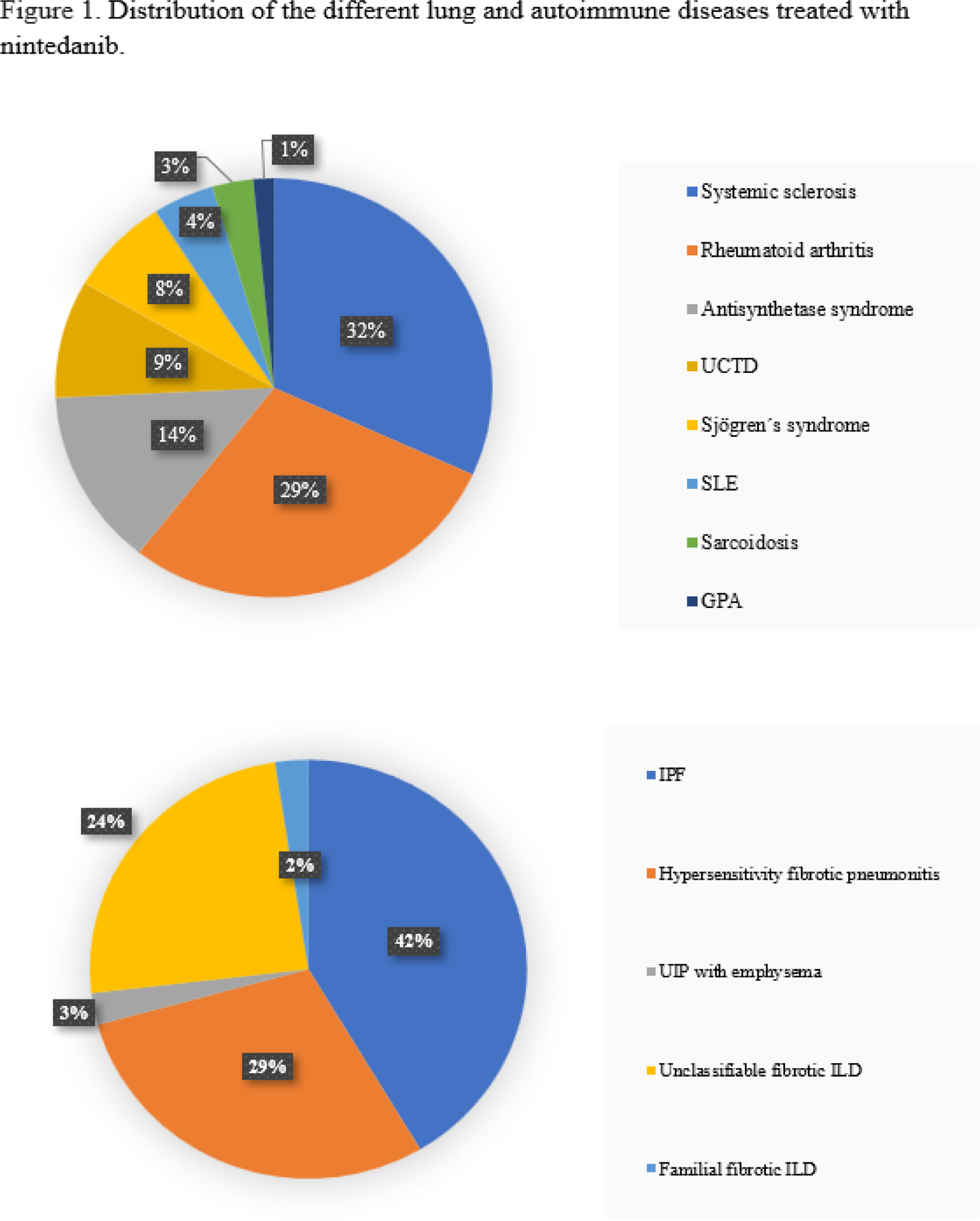
Results: Among the AD-ILDs and non-AD-ILDs group, 58% and 32% were women ( p=0.01 ). The distribution of the different pulmonary and autoimmune diseases is shown in Figure 1. Age at the time of NINTE administration was 63.7 (56.4-71.2) and 72.4 (66-78.4) years, respectively ( p<0.001 ) (Table 1). The median time from diagnosis of ILD to onset of NINTE was 3.6 (1.6-5.9) and 2.3 (1-4) years ( p=0.04 ). 24 patients had a history of occupational exposure (9% and 44%, p<0,001 ) (Table 1). The presence of emphysema on baseline thoracic high resolution computed tomography (HRCT) was more frequent in the control group ( p= 0.03 ), whereas ground glass was more frequent in cases ( p=0.02 ). Honeycombing and reticulation patterns were similar in both groups. Patients with AD-ILD had higher ESR ( p<0.001 ) and CRP ( p<0.001 ) levels before the initiation of NINTE. At the time of starting NINTE, mean FVC% predicted were 71.9 and 75.1; and mean DLCO% predicted were 45.8 and 41.4, respectively. Pulmonary function test remained stable across the first 12 months in AD-ILD and non-AD-ILD (FVC% 71.0 and 80%; DLCO% 47 and 37.9), Available thoracic HRCT images improved/stabilized in 74% and 78% of patients in the case and control groups, respectively. In both groups, adverse events, acute exacerbations of ILD and discontinuation rate were similar. During a median follow-up of 15 (8.6-29.5) months, 24 patients withdrew NINTE due to gastrointestinal adverse events and 30 subjects required a dose reduction (150 mg daily).
Conclusion: Baseline clinical and radiological characteristics between AD-ILD and non-AD-ILD were different, while respiratory function tests were similar. During follow-up, FVC%, DLCO% and HRCT images stabilized in both groups. Therefore, we conclude that NINTE may be effective for both AD-ILD and non-AD-ILD, although longer follow-up time would be needed to see the real efficacy of the drug.
Clinical, analytical, radiologic and functional characteristics of 108 patients treated with nintedanib1.
| AD-ILD (n 67) | Non-AD-ILD (n 41) | p value | |
|---|---|---|---|
| Female | 39 (58%) | 13 (32%) | 0.01 |
| Age at NINTE onset | 63.7 (56.4-71.2) | 72.4 (66-78.4) | <0.001 |
| Duration of ILD (years)
| 3.6 (1.6-5.9)
| 2.3 (1-4)
| 0.04
|
| ESR level, mm
| 14 (9-29.3)
| 10 (6-17)
| <0.001
|
| UIP pattern
| 22 (33%)
| 14 (34.1)
| NS
|
1Median (IQR), or number (%); 2Gastrointestinal adverse event.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.