

Background: There is a limited offer of fully-automated multiplexed devices for testing autoantibodies associated with connective tissue diseases (CTD).
Objectives: To assess the performance characteristics of the novel MosaiQ ® AiPlex CTD microarray immunoassay (AiPlex-CTD) when used with the fully-automated MosaiQ system, for simultaneous qualitative detection of eleven autoantibodies commonly found in CTD.
Methods: A comparator study was conducted at Hôpital Pitié-Salpêtrière (Paris, France), using anonymized human serum samples, characterized as non-reactive for all analytes (n= 445) or reactive for ≥1 analytes included in the assay (n= 466) with a composite of CE-marked devices used in routine laboratory practice, as part of AiPlex-CTD evaluations towards CE mark under IVDR. For dsDNA, samples were reactive by HEp-2000 ® -IgG-FLUORESCENT-ANA-Ro, (ANA-IFA) (ImmunoConcepts, USA) and Anti-dsDNA-IgG-ELISA (DRG, Germany). For other analytes, samples were reactive by ANA-IFA and FIDIS™-Connective-Profile (FIDIS) (Theradiag, France) or were reactive by ANA-IFA and/or ANAscreen (ANA-ELISA) (ORGENTEC-Diagnostika-GmbH, Germany) but FIDIS non-reactive or equivocal. Non-reactive samples were characterized with both ANA-IFA and ANA-ELISA.
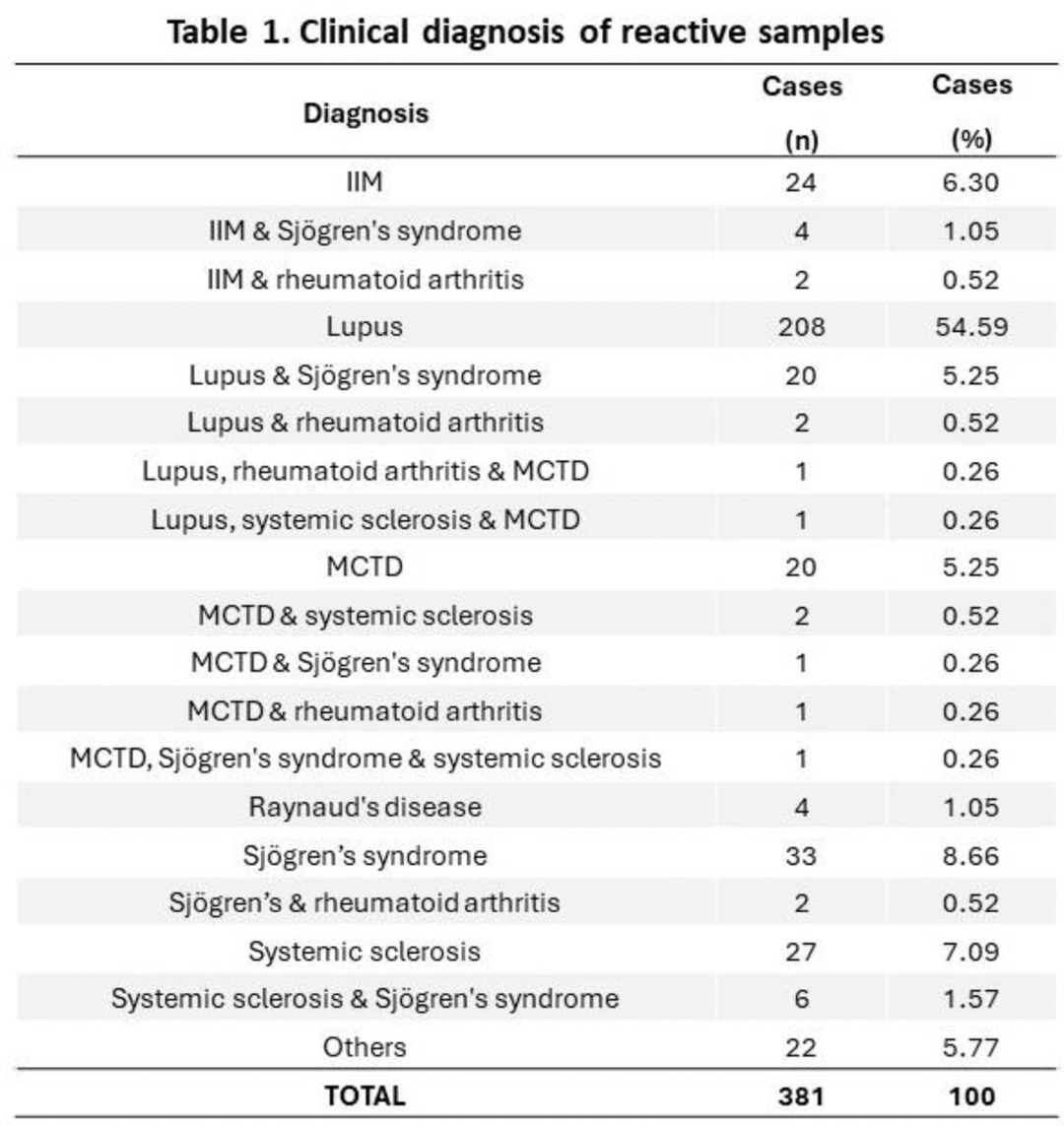
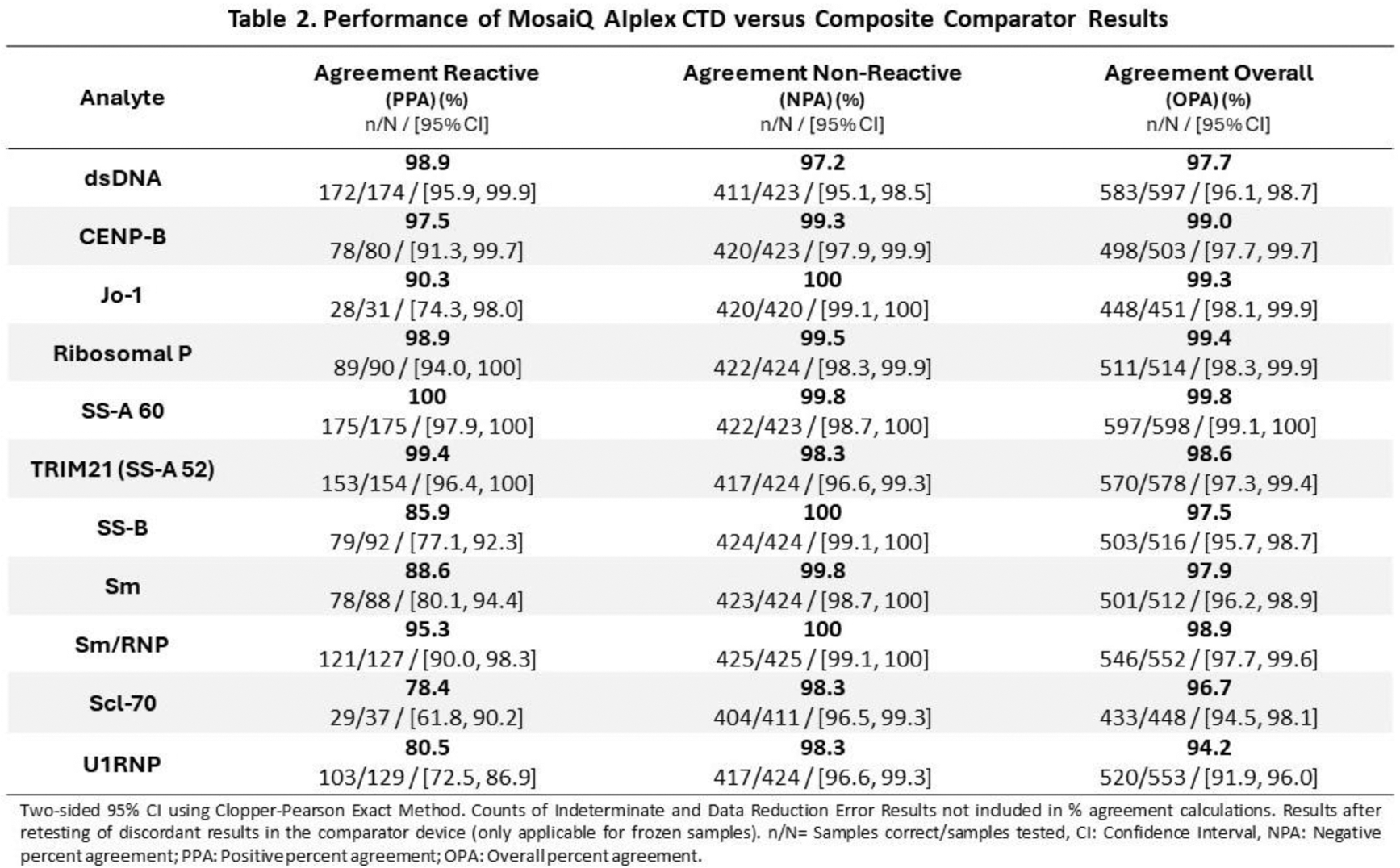
Results: Information on clinical diagnosis was available for 381 of the 466 enrolled reactive samples (Table 1). The most frequent diagnosis was lupus, followed by Sjögren’s syndrome, systemic sclerosis, idiopathic inflammatory myopathies (IIM), and mixed connective tissue disease (MCTD). In 43 samples (11.29%) the diagnosis featured more than 1 CTD. Other diseases included a range of conditions ranging from various autoimmune disorders to malignancies. After discordant analysis, compared with composite comparator results, AiPlex-CTD showed Positive Percentage Agreement (PPA) ranging from 78.4% for SCL-70 to 100% for SS-A 60. Negative Percentage Agreement (NPA) ranged from 97.2% for dsDNA to 100% for Jo-1, SS-B and Sm/RNP. Performance details for each individual analyte are shown in Table 2.
Conclusion: MosaiQ AiPlex-CTD demonstrated high concordance with compared CE-marked devices for the automated qualitative detection of the eleven autoantibodies included in the assay. This platform has the potential to contribute to the advancement of CTD testing. Further ongoing steps include expanding the number of printed antigens and semi-quantification.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Pascale Ghillani-Dalbin: None declared, Christel Daubrosse: None declared, Valerie Mercier: None declared, Daphne Bijlsma AliveDx, Ewa Lukasik AliveDx, Rocio Pasion Galvan AliveDx, Valeria Botti AliveDx, Gerber Gomez AliveDx, Emmanuel Moreau AliveDx, Michael Hausmann AliveDx, Christine C Ginocchio Dr. Ginocchio was employee of AliveDx at the time the study was conducted, Makoto Miyara participation in Scientific Advisory Board meetings for AliveDx.