

Background: Rheumatic and musculoskeletal diseases (RMDs) encompass a variety of chronic conditions, which most often affect women of childbearing age. Most RMDs require long-term treatment with disease modifying anti-rheumatic drugs (DMARDs): Disease quiescence is vital to reduce complications in pregnancy in the mother and the offspring. Clinical guidance documents to inform on the compatibility of DMARDs with pregnancy often differ from regulator advice. This assessment of regulator advice was designed to uncover potential discrepancies regarding the use of DMARDs in pregnant patients with RMDs.
Objectives: Our aim is to provide a systematic overview of reference safety documents content for the use in the pregnancy and present the interim analysis from seven major regulators.
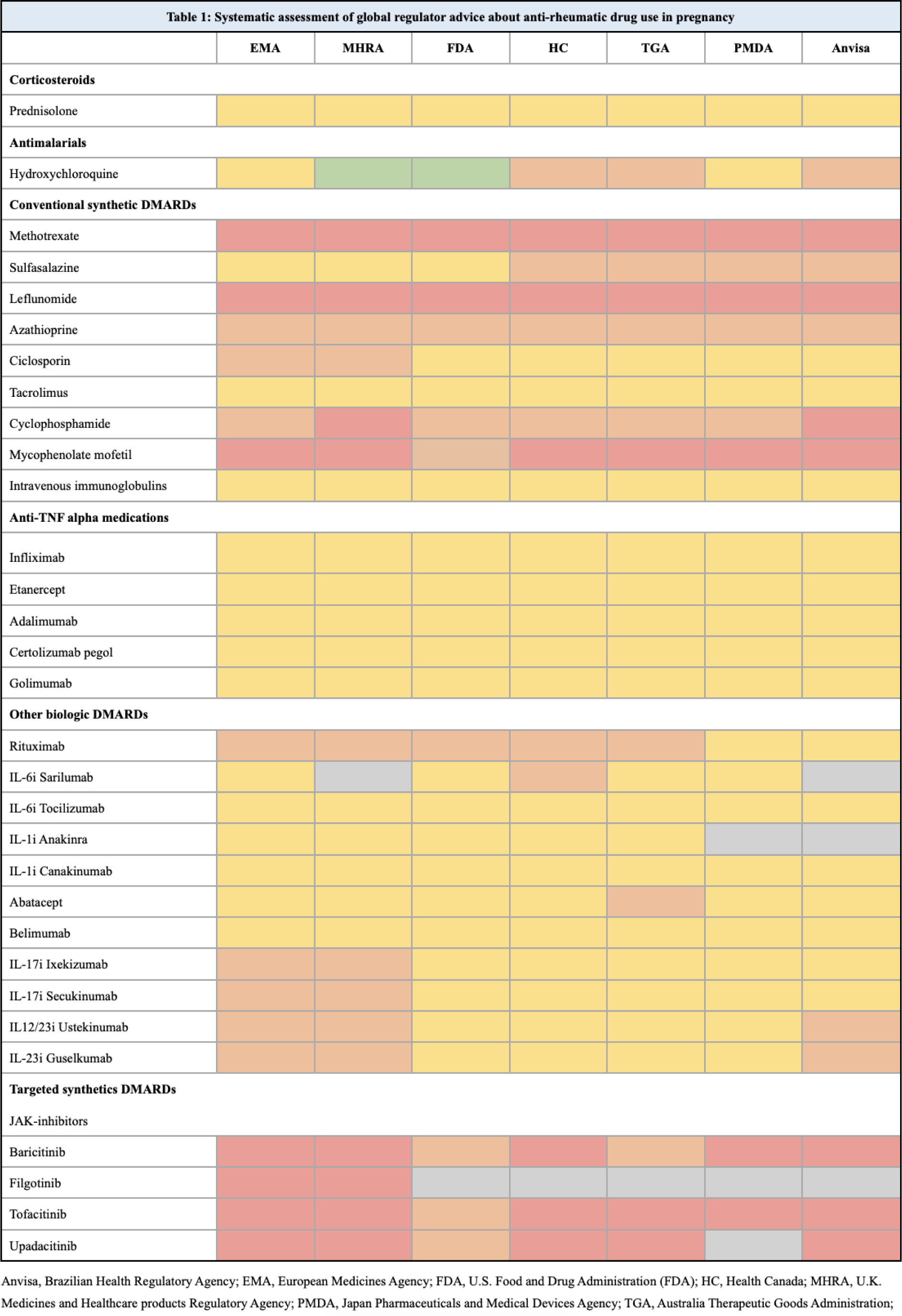
Methods: A systematic assessment of reference safety information for anti-rheumatic medications from seven international medical authorities was performed. Full information about pregnancy compatibility on all relevant DMARDs was identified on the website of the different authorities. Native speakers translated the information from Portuguese and Japanese. The information about the use of each drug during pregnancy was extracted and classified individually by two reviewers, and a third reviewer solved discrepancies. The classification was made on the basis of interpretation of the information and categorized into A-D as follows: Category A (green): Compatible with pregnancy; Category B (yellow): OK to use during pregnancy if needed; Category C (orange): OK to use during pregnancy if no other options; Category D (red): Contraindicated. Medication not approved by a medical authority is marked in grey (Table 1).
Results: Thirty-one individual DMARD safety reference documents were accessed covering the following authorities: European Medicines Agency (EMA), U.K. Medicines and Healthcare products Regulatory Agency (MHRA), U.S. Food and Drug Administration (FDA), Health Canada (HC), Australia Therapeutic Goods Administration (TGA), Japan Pharmaceuticals and Medical Devices Agency (PMDA), and Brazilian Health Regulatory Agency (Anvisa). The total population covered by these regulatory agencies is app. 1.246 billion people, representing app. 15.4% of the global population. Four medications had not been regulator approved (for any use) by all seven authorities. Category A was granted only to hydroxychloroquine by two medical competent authorities (FDA and MHRA). Fifteen medications were categorized inconsistently by the different authorities and 16 medications were categorized equivalently.
Conclusion: Information on the compatibility of using DMARDs during pregnancy is interpreted differently in nearly half of the most common medications used in RMDs. The discrepancies in regulator advice on the use of DMARDs in pregnancy among international medical authorities may lead to confusion for healthcare providers and patients, may hinder international research projects, it may influence treatment decisions and the management of RMDs during pregnancy, with potential consequences for maternal and fetal health.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.