

Background: Detection of various relevant autoantibodies plays an important role in the identification of connective tissue diseases (CTD); however, there is currently a limited offer of automated multi-analyte tests, and many currently available methods are either single tests and/or manual or semi-automated. The development of fully automated multiplexed devices remains a need.
Objectives: To assess the analytical performance of a novel, single-use, multiplexed microarray immunoassay prototype (MosaiQ AiPlex CTDplus) when used with the fully automated MosaiQ® system, for the simultaneous detection of fifteen autoantibodies associated with connective tissue diseases (CTD), compared with selected CE-marked devices.
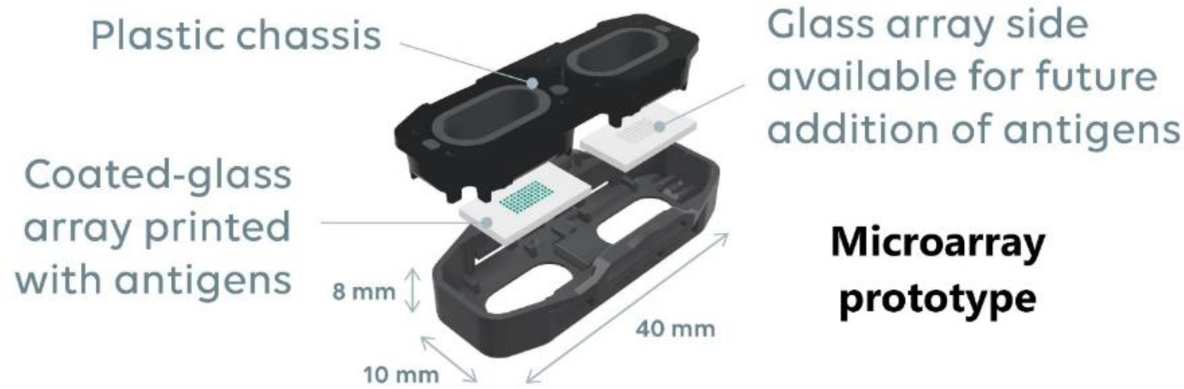
Methods: Microarray prototypes (AliveDx, Eysins, Switzerland) (Figure 1) have two separate sides of functionalized glass of which one was printed with antigens (Figure 1). Microarrays are assembled into magazines for processing on the MosaiQ 125 instrument. Human serum samples in primary tubes, buffers and reagents are loaded on the instrument, then microarrays are automatically processed, generating immunoassay spot signals that are interpreted by the instrument using a proprietary algorithm for image analysis. The system includes security features such as radiofrequency identification (RFID) and when magazines and reagents are loaded on the instrument, key information (i.e., lot number, expiry date and volume) is transmitted to it to assess whether resources loaded are adequate to perform the required test order. In addition to antigens, every microarray contains internal positive and negative controls to identify the location of the printed antigen probes to enable processing of the image, addition of reagents and to demonstrate that no non-specific results occur due to the print matrix. Banked human serum samples, characterized as reactive to ≥1 autoantibody or non-reactive were included. Reactive samples were characterized with FIDIS TM Connective Profile (Theradiag, France) for CENP B, Jo-1, Ribosomal P, Scl-70, Sm, Sm/RNP, SS-A 60, TRIM21 (SS-A 52), SS-B and U1RNP; EliA CCP (Phadia, Germany) for CCP2, QUANTA Lite® Chromatin (Werfen, Spain) for Chromatin, QUANTA Flash® DFS70* (Werfen, Spain) for DFS70, Anti-dsDNA-IgG-ELISA (DRG, Germany) for dsDNA, and Immunodot (Euroimmun, Germany) for RNA polymerase III (RNAP III). For CCP2, only reactive samples were included. For the remaining analytes, non-reactive samples were characterized using both immunofluorescence (ANA-Ro IgG FLUORESCENT HEp-2000®; Immuno Concepts, Germany) and ANAscreen ELISA (ORGENTEC-Diagnostika-GmbH, Germany). Each individual non-reactive sample was tested with the investigational device once and reactive samples were tested in duplicates. Positive percent agreement (PPA) and negative percent agreement (NPA) for individual analytes were calculated. For CCP2 only PPA is presented as no negative samples were included.
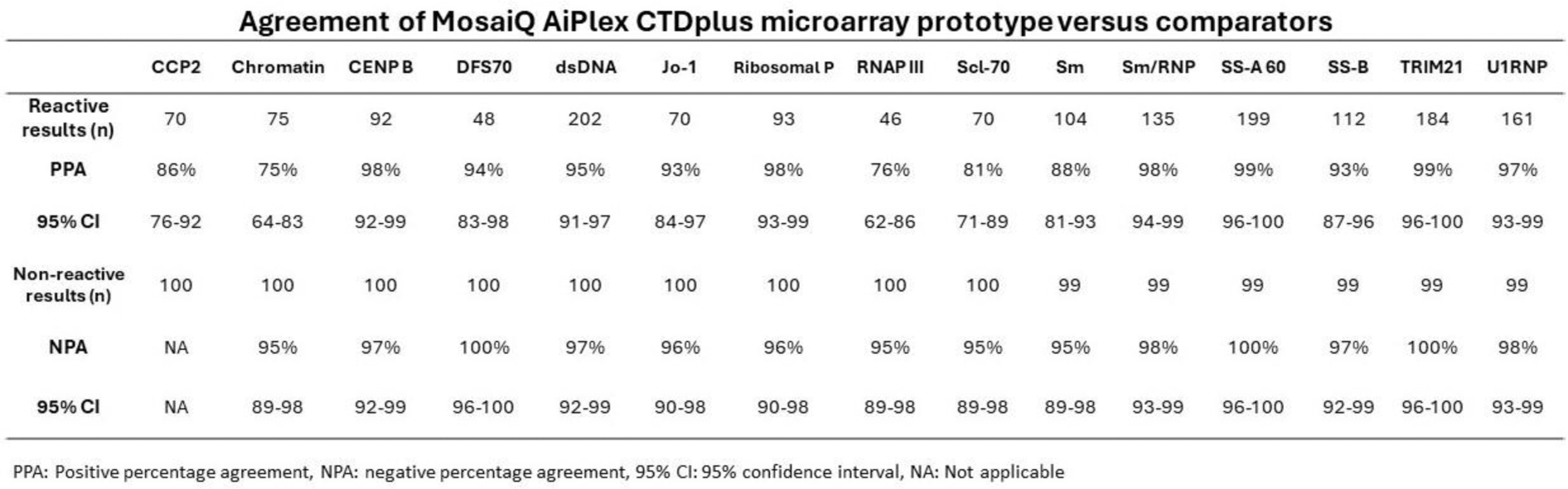
Results: Compared with relevant devices, the investigational prototype showed positive percentage agreement (PPA) ranging from 75% for chromatin to 99% for SS-A 60 and TRIM21. Negative percentage agreement (NPA) ranged from 95% for chromatin, RNAP III, Scl-70 and Sm to 100% for DFS70, SS-A 60 and TRIM21. Performance of individual analytes is shown in the Table 1.
Table 1.
Conclusion: The investigational prototype showed preliminary results demonstrating substantial agreement with the compared CE-marked devices for the detection of the autoantibodies included in the assay. Further analytical studies with the final prototype and evaluation of samples with clinical diagnosis will allow for expanded performance assessment of the investigational device. This fully automated multiplexed platform has the potential to contribute to optimizing CTD evaluation by simplifying complex testing pathways and analyzing large number of samples per day.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Daphne Bijlsma AliveDx, Ewa Lukasik AliveDx, Michael Hausmann AliveDx, Gerber Gomez AliveDx, Christian Fischer AliveDx, Pascale Ghillani-Dalbin: None declared, Makoto Miyara participation in Scientific Advisory Board Meetings for AliveDx, Emmanuel Moreau AliveDx.