

Background: Glucand fragility fractures are common comorbidities in patients with rheumatic and musculoskeletal diseases (RMD) such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Although anti-resorptive and anabolic agents are recommended to reduce fracture risks in GIO, their effects on controlling the underlying disease activity of RMD have received less attention. Previous research suggests that inhibiting RANK with denosumab (DMAB) may have immunosuppressive effects. Teriparatide (TPD) and romosozumab (ROMO) are two drugs about which little is known.
Objectives: To compare disease activity control across groups of RMD patients with GIO receiving osteoporosis treatment with denosumab, teriparatide, romosozumab, or zoledronic acid (ZA).
Methods: This prospective cohort study included patients from a specialty osteoporosis clinic with RMDs who began osteoporosis therapy in the years 2021-2022. The primary outcome was disease activity at the next clinical assessment for RMD. To account for differences in several activity measures, disease activity was homogenised on an ordinal 3-point scale (remission, low, moderate/high) at baseline and follow-up according to a independent assessor. The analysis of variance was used to compare disease activity score changes an ross groups over time, while controlling for baseline fracture risk assessment tool (FRAX).
Results: During the study period, 99 RMD patients were given one of the study medications, and 44 of them had a follow-up evaluation at a median of 6.8 (range 2.7 to 13.6) months and were analysed (DMAB: n = 11, TPD/ROMO: n = 13, and ZA: n = 20). At baseline (Table 1), the majority of patients (55%), had chronic inflammatory arthritis, had taken oral bisphosphonates, had vertebral fractures, and were at high fracture risk. Follow-up disease activity scores decreased by -0.2 points for ZA, -0.5 points for DMAB, and increased by 0.4 points for TPD/ROMO groups (Figure 1), with only the DMAB group showing significant between-group differences in improvement over follow-up (mean difference -0.8, 95% CI -1.6 to -0.02, p=0.043). However, this was not evident in the adjusted analysis, which included baseline FRAX as a covariate.
Baseline descriptives (unmatched)
|
ZA
| DMAB (n=11 ) |
TPD/ROMO
| p-value | |
|---|---|---|---|---|
| Age, mean (SD) | 66 (10) | 67 (10) | 69 (8) | 0.796 |
| Female (%) | 85 | 81.8 | 92.3 | 0.861 |
| Prior bisphosphonate use (%) | 50 | 27.3 | 61.5 | 0.238 |
| Baseline spine BMD T-score, mean (SD) | -2.3 (1.3) | -2.2 | -1.6 (0.5) | 0.351 |
| Baseline femoral neck BMD T-score, mean (SD) | -2.0 (1.5) | -1.3 (1.4) | -0.6 (0.9) | 0.109 |
| FRAX 10-year risk, mean (SD) | 32 (16) | 23 (10) | 62 (28) | 0.009 |
| Baseline vertebral fractures (%) | 45 | 18 | 100 | <0.001 |
| RMD (%) | ||||
| CIA (RA, PsA, SpA) | 65 | 36 | 54 | 0.177 |
| CTD (SLE, SSc, SS) | 5 | 18 | 31 | |
| Systemic vasculitis (AAV, PAN, GCA) | 30 | 46 | 15 | |
| Glucocorticoid therapy (%) | 100 | 86 | 100 | 0.444 |
| Prednisone dose, mg (SD) | 4.7 (0.6) | 5.8 (5.0) | 6.7 (1.2) | |
| Disease activity (%) | ||||
| Remission | 40 | 55 | 62 | 0.008 |
| Low | 15 | 0 | 38 | |
| Moderate and high | 45 | 45 | 0 |
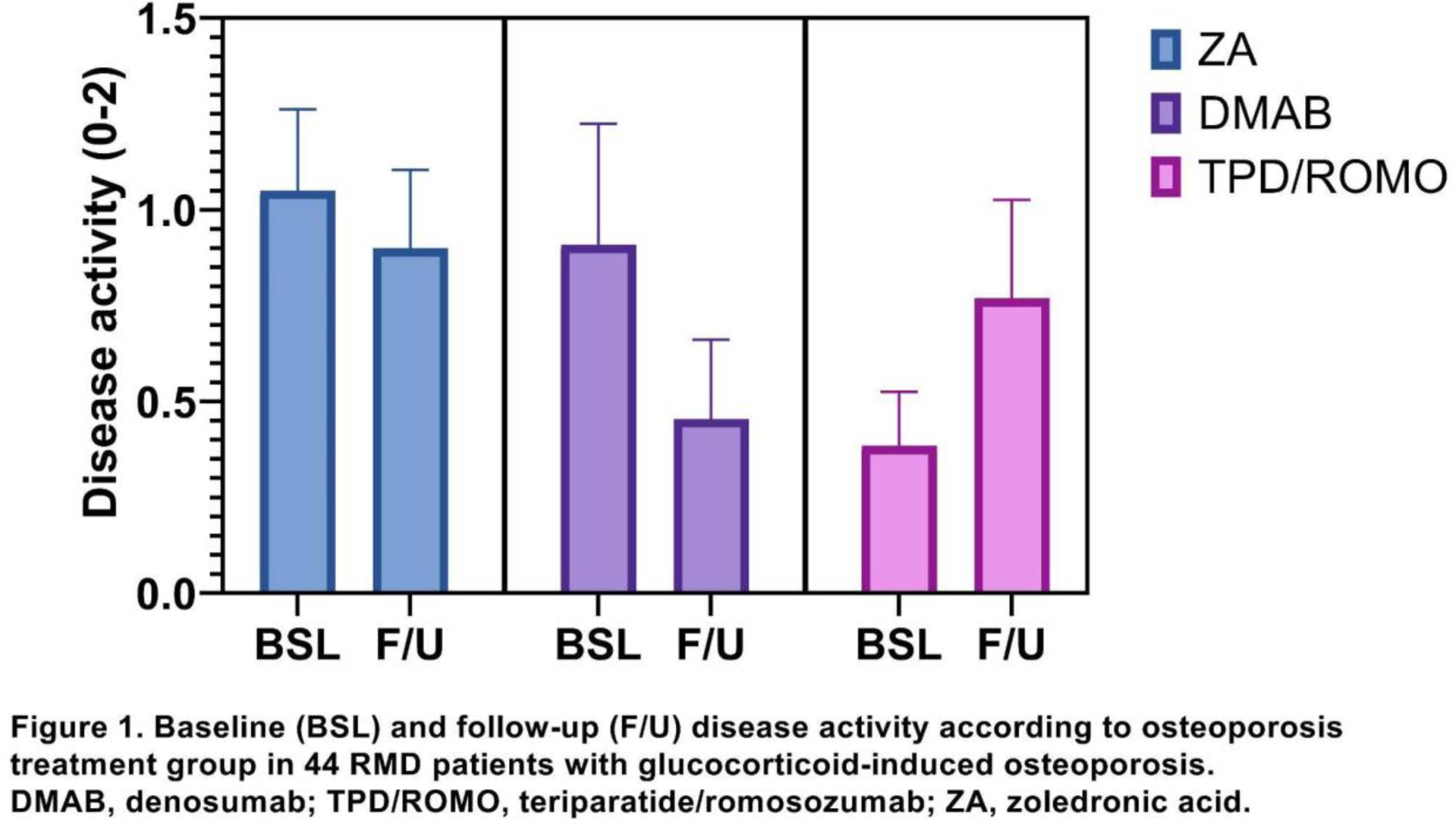
Conclusion: In this small sample, there were no statistically significant differences between osteoporosis medications in disease activity improvement among patients with inflammatory rheumatic diseases and GIO. Fracture risk might mediate the effect of osteoporosis medications and disease activity control. Larger pragmatic trials are warranted.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.