

Background: Biosimilar biological disease modifying anti-rheumatic drugs (B-bDMARDs) are a cost-effective treatment for autoimmune rheumatic diseases (ARDs). Despite current guidelines [3] consider them as equal as originators, clinical data about the use of B-bDMARDs during pregnancy are limited [1, 2], so more data are needed to support the same pattern of use during pregnancy as for originators (O-bDMARDs).
Objectives: To describe maternal disease course and obstetric/neonatal outcomes in a monocentric cohort of women with ARDs treated with B-bDMARDs during pregnancy.
Methods: This prospective study described 14 pregnancies in 13 women occurring during B-bDMARDs treatment from 2020. Data about drug adverse events, disease flares, comorbidities, obstetric and neonatal outcomes were collected from clinical charts.
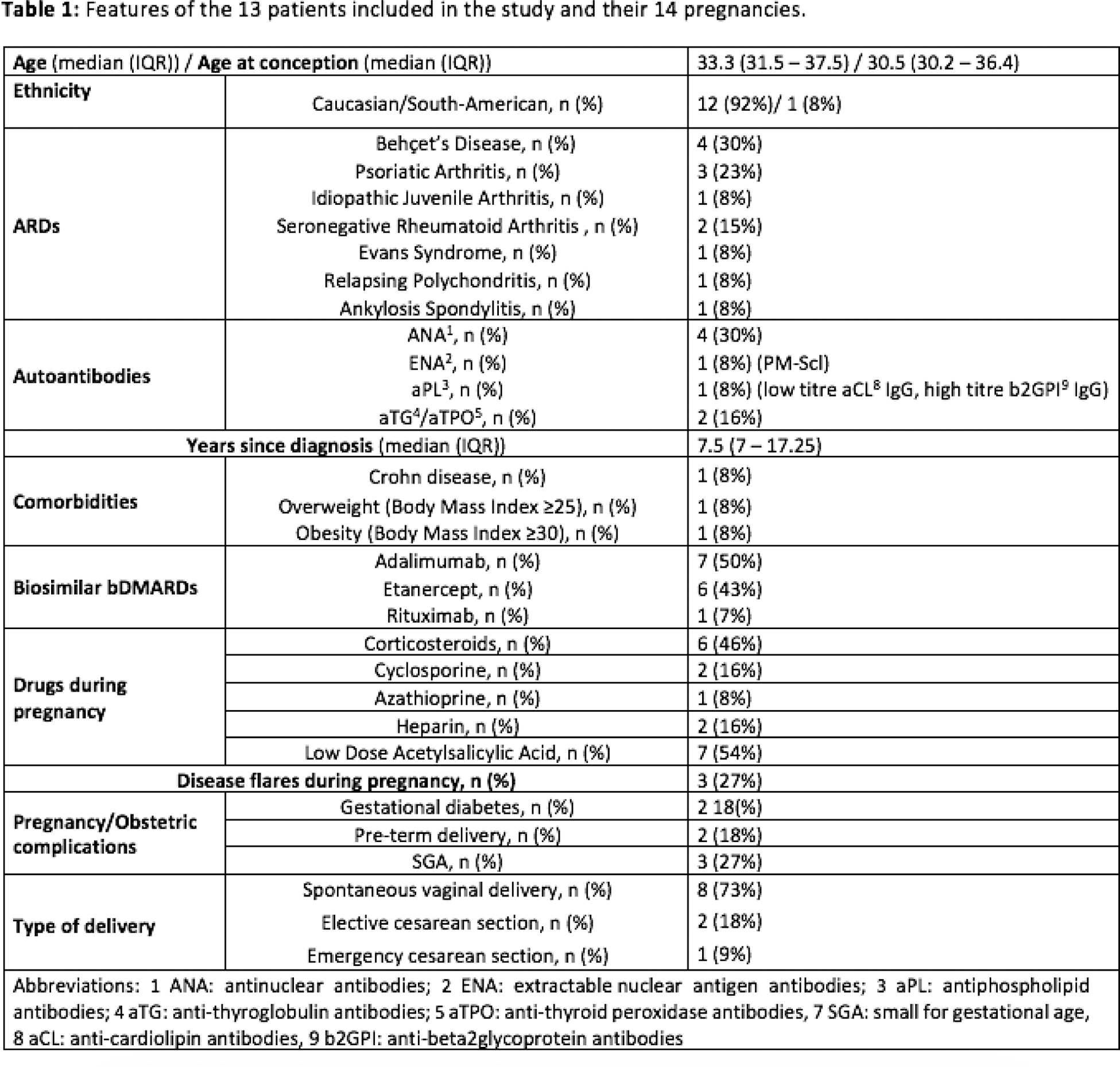
Results: Of 13 women, 10 were primigravidae. B-bDMARDs treatment was already ongoing in the preconception period in 11 pregnancies (median treatment duration 6 years, IQR 3-7), whereas it was established during pregnancy in 4 pregnancies. To date, 3 patients are still pregnant and no gestational complications have occurred. Among the 11 pregnancies concluded, one was a twin pregnancy. At conception, only the patient with Evan’s syndrome wasn’t in clinical remission (severe autoimmune thrombocytopenia) and she was off-therapy. Her pregnancy required a rituximab cycle during the second trimester, in association with 4 cycles of intravenous immunoglobulins, 2 blood transfusions, cyclosporine and high dosage of intravenous corticosteroids (GC). She delivered at 34 + 7 gestational week (GW) with elective cesarean section (CS) a small gestational age (SGA) baby (birth weight (BW): 1900 g) who required neonatal intensive care for 10 days. Regarding TNF-inhibitors, 4 patients stopped adalimumab (ADA) during the second trimester (median 24 GW, IQR 21 - 24.75) and 5 stopped etanercept (ETA) during the third trimester (median 32 GW, IQR 31-34). A woman with relapsing polychondritis continued ETA throughout gestation due to the risk of disease flare. Concerning flares, a woman with Juvenile Idiopathic Arthritis (JIA) who discontinued ETA preconceptionally for clinical remission restarted it during the first trimester because of a flare of arthritis. Another woman with Behcet’s disease developed a Crohn’s disease flare during the third trimester after discontinuation of ADA at positive pregnancy test. These two flares were mild and treated with medium-low dose GC (≤10mg/die). Concerning obstetric complications, 2 women developed gestational diabetes (both were under GC treatment, one was obese). Delivery occurred at a median GW of 37.7 (IQR 36.2 – 39.2) and babies had a median birth weight of 2800 g (IQR 2297.5 - 3150). Besides the patient with Evan’s syndrome, preterm delivery was observed in a patient with JIA who delivered a SGA baby (BW: 2060 g) at 35 GW. Two women delivered by CS, of which one was an emergency CS in a woman pregnant with twins who developed doppler velocimetry alterations (36 GW) and one was an elective CS due to placenta previa (37 GW). No other neonatal or obstetric complications occurred. Out of 11 women who had delivered a live baby, 4 did not breastfeed (2 for personal choice, 2 for agalactia), while 7 did (6 were on treatment with B-bDMARDs). Nine women (82%) restarted B-bDMARDs within 6 months after delivery, in 3 cases due to arthritis, in 6 cases as part of the scheduled restart of B-bDMARD. The features of the 13 patients are summarized in Table 1.
Conclusion: B-bDMARDs use during pregnancy did not appear less effective or safe as O-bDMARDs in our case series. Particularly, no congenital abnormalities nor perinatal complications occurred. Discontinuation at positive pregnancy test or a delayed start during pregnancy of B-bDMARDs seems to be associated with disease flares and preterm birth. Therefore, as recommended for O-bDMARDs, we suggest considering B-bDMARDs use beyond conception and throughout pregnancy if needed to keep maternal disease under control.
REFERENCES: [1] DOI:10.1177/1753495X211028779.
[2] DOI:10.1093/ecco-jcc/jjx180.741).
[3]
Acknowledgements: NIL.
Disclosure of Interests: None declared.