

Background: Autoreactive B cells are crucially involved in the pathogenesis of autoimmune rheumatic diseases (ARD) and represent a classic treatment target. However, conventional B cell depletion with rituximab does not address tissue-resident long-lived plasma cells and is associated with insufficient treatment response in a subset of patients. Consequently, plasma cell-targeting therapies could represent an option in cases with life- or organ-threatening manifestations. In this context, anecdotal reports described promising effects of daratumumab, a monoclonal antibody directed against CD38 that depletes plasma cells [1].
Objectives: To describe the clinical efficacy and safety of daratumumab in six patients with ARDs.
Methods: Six ARD patients, refractory to B-cell-depleting agents as well as various other immunosuppressive agents, received daratumumab as a rescue therapy. Disease activity parameters were assessed before treatment initiation and during the follow-up period. All patients gave written consent to this off-label therapy and anonymous publication of the data.
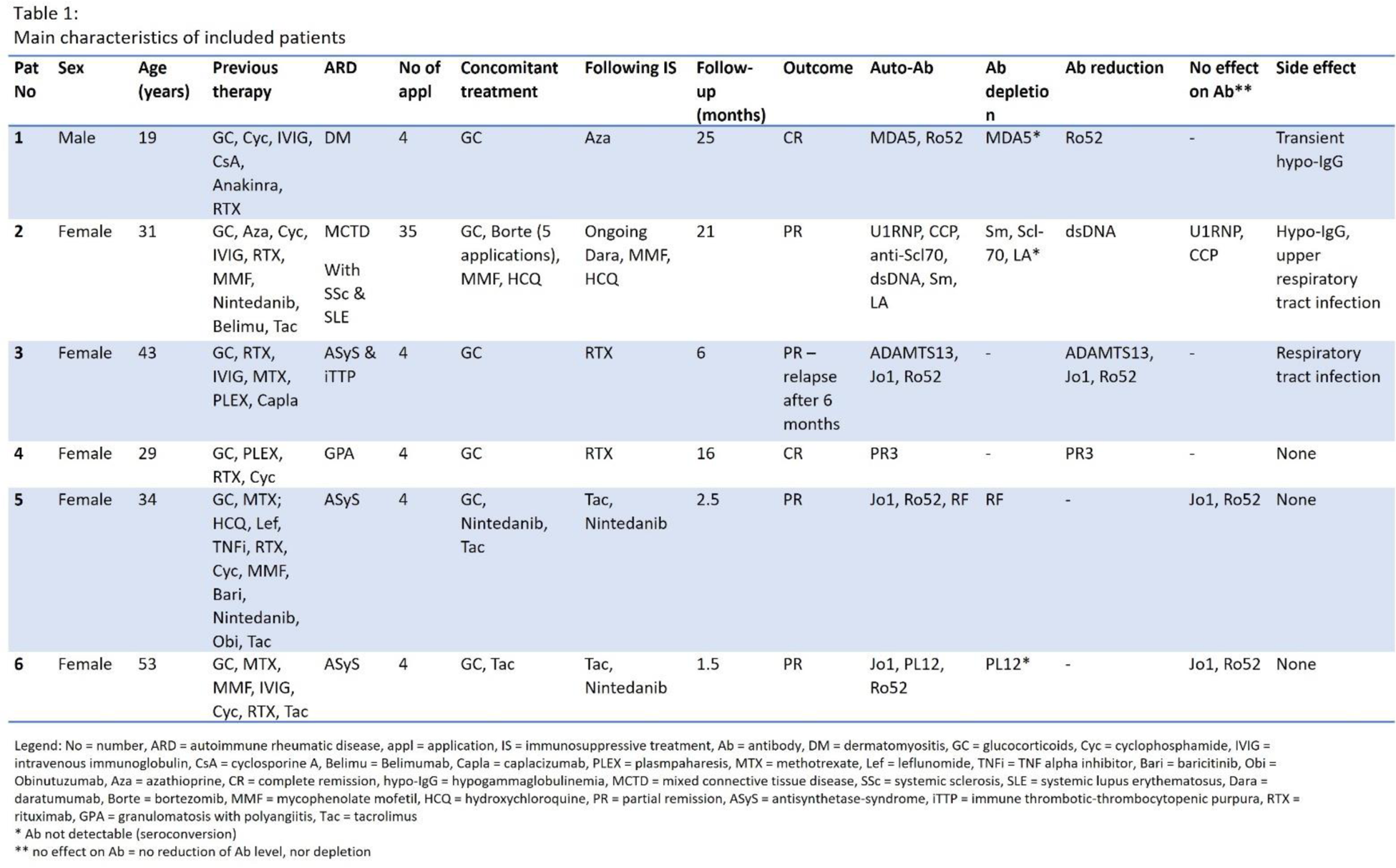
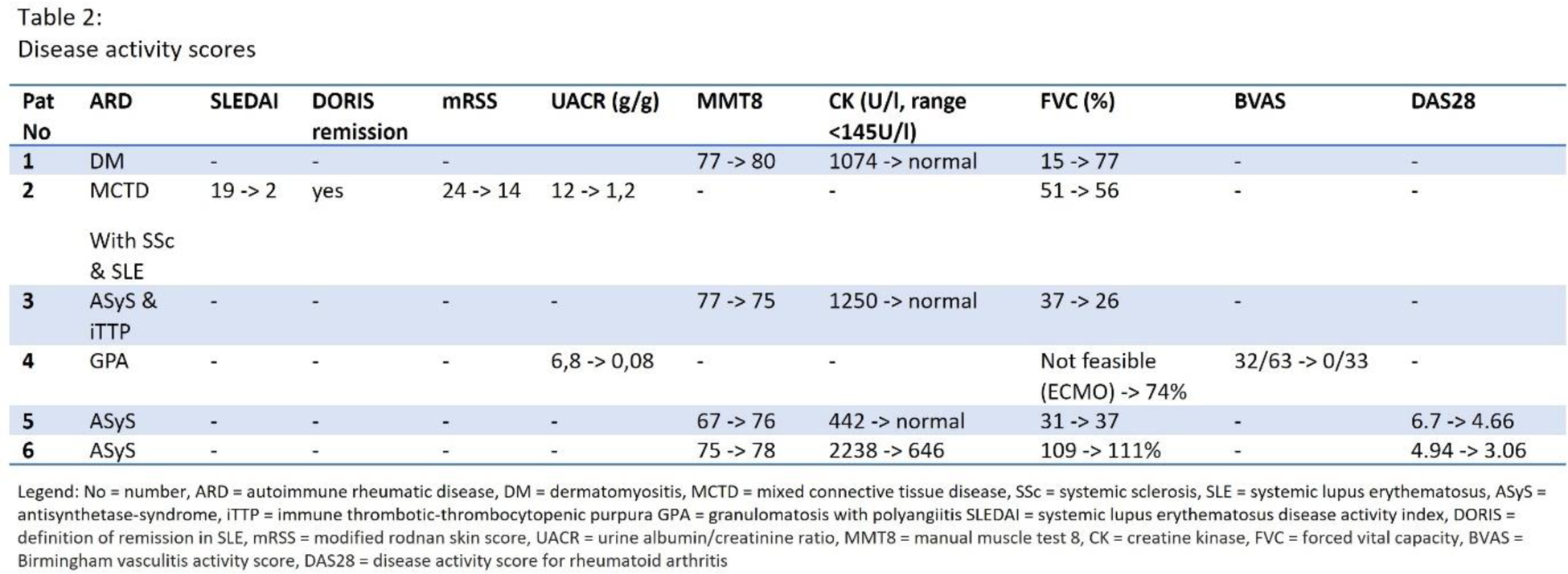
Results: The six patients were mainly female (83%) with a median age of 35 years. Four myositis patients with interstitial lung disease (three Jo1-antisynthetase-syndrome (Jo1-ASyS) patients and one MDA5 positive dermatomyositis patient), one granulomatosis with polyangiitis with rapid-progressive glomerulonephritis and pulmonary hemorrhage as well as one patient with mixed connective tissue disease (MCTD) with lupus nephritis, interstitial lung disease and diffuse cutaneous systemic sclerosis (dcSSc) were treated with daratumumab. All patients were refractory to various immunosuppressants including rituximab. Two patients (33%) showed stable complete response over a follow-up period of 25 and 16 months respectively. Three patients (50%) achieved partial response with a follow-up period ranging from 1.5 months to 21 months. One patient with Jo1-ASyS and immune thrombotic-thrombocytopenic purpura had a relapse after partial remission 6 months after last daratumumab application and will receive another cycle of daratumumab. Autoantibody depletion or reduction was seen in half of the patients. Five patients (83%) received four applications of daratumumab subcutaneously (1800mg/week), whereas the one patient with MCTD showed persisting disease activity after initial improvement under pausing of daratumumab. Continuation of daratumumab according to the multiple myeloma treatment regimen (on a now monthly basis) paralleled by five applications of bortezomib lead to improvement of lung, renal and skin involvement (see Tables 1 and 2). No injection side reactions were noticed, two patients experienced respiratory infections without further complications, one patient developed transient hypogammaglobulinemia and one patient has persistent hypogammaglobulinemia (see Table 1).
Conclusion: Daratumumab is a promising therapeutic approach in refractory cases of ARD with organ- or life-threatening manifestation. No severe adverse events were noticed with continuous infection prophylaxis with cotrimoxazole and acyclovir. The treatment regimen varied depending on the treatment response. Further studies are warranted to evaluate the optimal treatment regimen, clinical efficacy, and safety of daratumumab in ARD.
REFERENCES:
[1] Holzer MT, Ruffer N, Huber TB, Kötter I, Ostendorf L, Krusche M. Daratumumab for autoimmune diseases: a systematic review. RMD Open. 2023 Dec 14;9(4):e003604. DOI: 10.1136/rmdopen-2023-003604.
Acknowledgements: NIL.
Disclosure of Interests: Marie-Therese Holzer Boehringer Ingelheim, Ina Kötter Lilly, Tingting Xiong Novartis, GSK, AstraZeneca, Nikolas Ruffer: None declared, Frederic Christian Feindt: None declared, Simon Melderis: None declared, Oliver Michael Steinmetz Novartis, DFG, Tobias B Huber: None declared, Martin Krusche Novartis, Abbvie, Lilly, Galapagos, Pfizer, Medac, GSK, Janssen, Novartis, Roche, Abbvie, Lilly, Galapagos, Pfizer, Medac, Novartis, Sobi, Sanofi.