

Background: Immune checkpoints inhibitors (ICI) cancer immunotherapy target specific molecules of the immune system to block the mechanisms that regulate the immune tolerance, enhancing the immune system’s response against tumor cells. Although these treatments have acceptable safety profiles, an increase in the occurrence of immune-related adverse events (irAEs) has been identified. IrAEs can affect any organ and system, with a wide range of manifestations and severity. Regarding rheumatic manifestations (R-irAEs), arthritis, myositis, polymyalgic syndrome, and others are being reported.
Objectives: Our aim was to characterize a cohort of patients treated with ICIs that develop R-irAEs.
Methods: An observational, descriptive study, including 734 patients treated with ICIs at Parc Taulí and Marqués de Valdecilla University Hospitals, between 2016 and 2022. Medical records were reviewed by a rheumatologist to identify possible manifestations compatible with R-irAEs.
Results: 734 patients treated with ICIs were analyzed. 227 (30.9%) presented irAEs, of which 54 (7.35%) were R-irAEs, (29 male/26 female), with an average age of 64.6±11.5 years at the starting of ICI treatment. Only 4 patients presented previous autoimmune diseases. Underlying cancer types included lung (n=24), melanoma (n=18), colorectal (n=4), renal (n=3), urothelial (n=3), gastric and hepatic (n=1), and oral cavity (n=1). 50 patients received ICIs as monotherapy. Average time from starting ICI treatment to onset of R- irAE was 18.0±35.6 weeks.
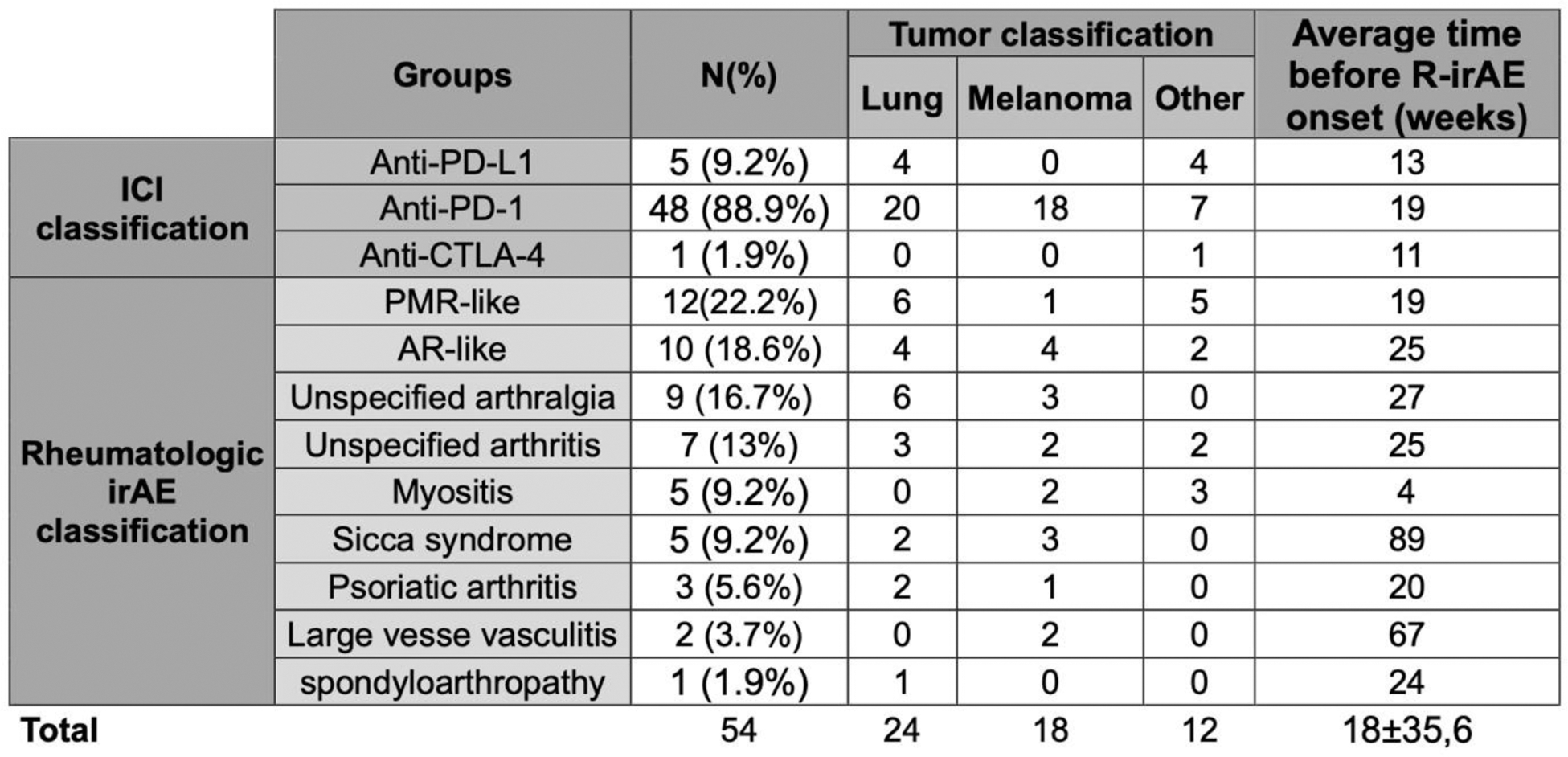
Clinically, patients presented as PMR-like (n=12), AR-like (n=10), unspecified arthralgia (n=9), unspecified arthritis (n=7), myositis (n=5), sicca syndrome (n=5), psoriatic arthritis (n=3), large vessel vasculitis (n=2), spondylarthritis (n=1). Demographic and clinic characteristics are summarized in Tables 1 and 2.
Elevated C-reactive protein (CRP) was detected in 18/21 patients, and altered erythrocyte sedimentation rate (ESR) in 8/10 patients. Autoantibody testing was positive in 11/40 patients, being the most frequent ANA positive (n=5; 3 with homogeneous pattern).
38 patients received corticosteroids, of those, 3 also required immunosuppressive (IS) synthetic drugs, 2 required IS biologic drugs and 4 required intravenous immunoglobulins. 27/39 responded favorably, with improvement in symptoms and no relapses. 18 patients had to stop their ICI treatment due to their R-irAEs.
Conclusion: The increasing use of ICIs in cancer treatment has led to an increase in irAEs. Moreover, irAEs (30.9%) and R-irAEs (7.35%) are relatively frequent. The most common R-irAEs are PMR-like (21.8%) and AR-like syndromes (18.2%). Analytically R-irAEs are characterized by high CPR (85%) and/or ESR (80%) and negative immunological tests. Although many patients respond favorably to treatment, 18 (33.3%) patients had to stop their ICI due to R-irAEs. Myositis appears as a characteristic disease, with faster onset, higher morbimortality and the need for intensive treatment, as seen in previous studies. More studies are needed to assess the long-term outcomes in these patients and the impact of IS treatment on cancer survival.
REFERENCES: NIL.
Table 1. Demographic and clinic data of ICI treated patients that develop R-irAEs.
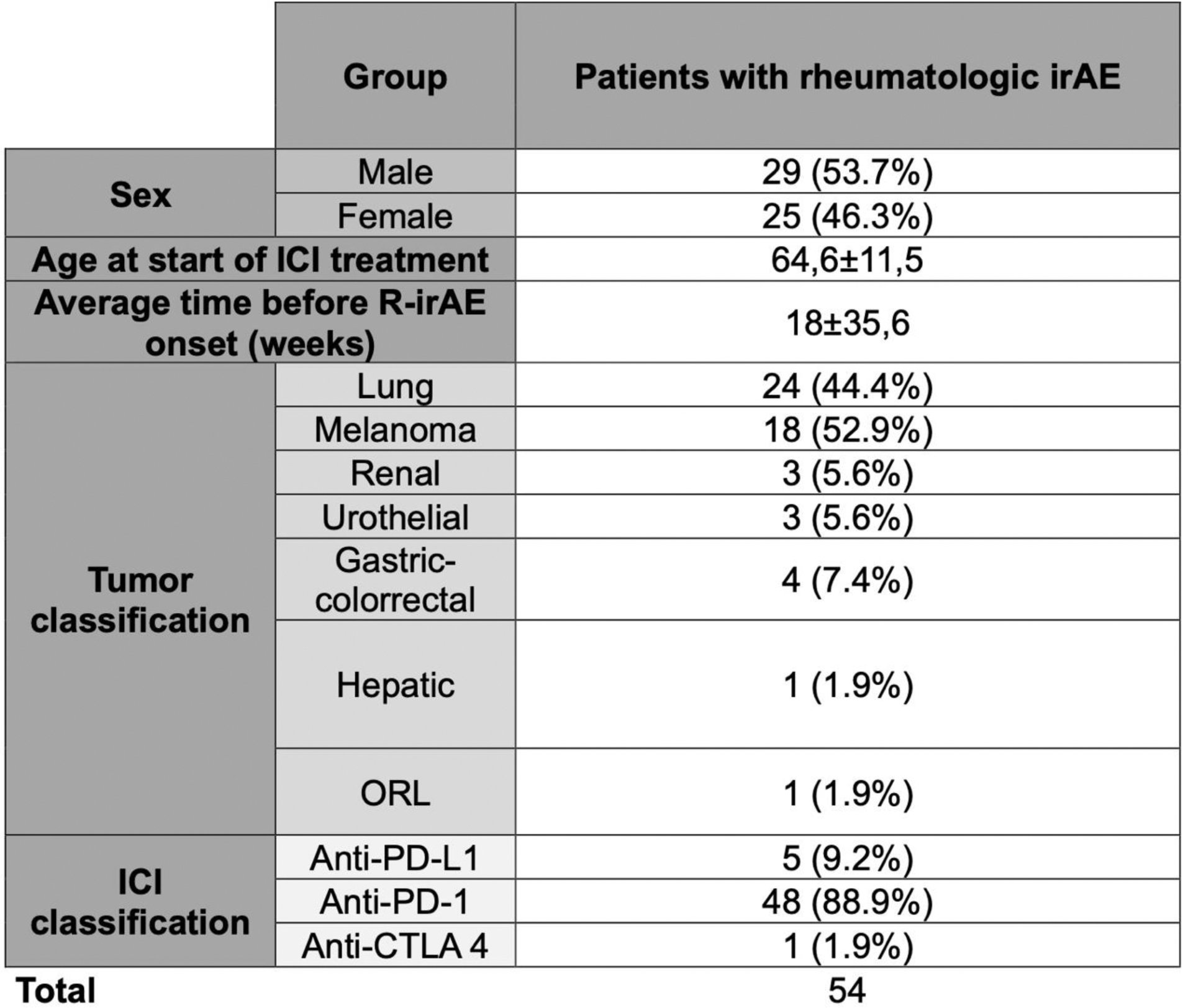
Table 2. Clinical characteristics of patients with rheumatologic irAEs.
Acknowledgements: NIL.
Disclosure of Interests: None declared.