

Background: Rheumatoid Arthritis (RA) and Psoriatic Arthritis (PsA) are immune-mediated rheumatic diseases for which multiple therapeutics are available. The Treatment Satisfaction Questionnaire for Medication (TSQM Version II) is a validated self-administered 11-item clinical outcome assessment questionnaire developed to assess patient satisfaction with their medications across four domains: Effectiveness (0-100), Side Effects (0-100), Convenience (0-100) and Global Satisfaction (0-100).
Objectives: To assess treatment satisfaction for medications among patients with RA and PsA in a tertiary rheumatology clinic using the TSQM Version II.
Methods: Patients were eligible if they were aged ≥18 years, on medication(s) for RA or PsA, and able to complete an English-language paper-based survey. Consecutive consenting patients seen in the Rheumatology outpatient clinics at Monash Health completed the TSQM Version II for each their arthritis medications. Demographics (age, gender, diagnosis, disease duration, patient-reported disease severity [0-10], current and previous medications) were collected and analysed using descriptive statistics. Associations between baseline demographics and patient satisfaction domains were assessed using the Mann-Whitney U test and Spearman’s rho where appropriate. Differences in patient satisfaction with conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), biological DMARDs (bDMARDs), targeted synthetic DMARDs (tsDMARDs) and prednisolone were assessed using the Kruskal Wallis test. Data analysis was completed using SPSS v.29. Monash Health Ethics Reference: RES-23-0000-498W
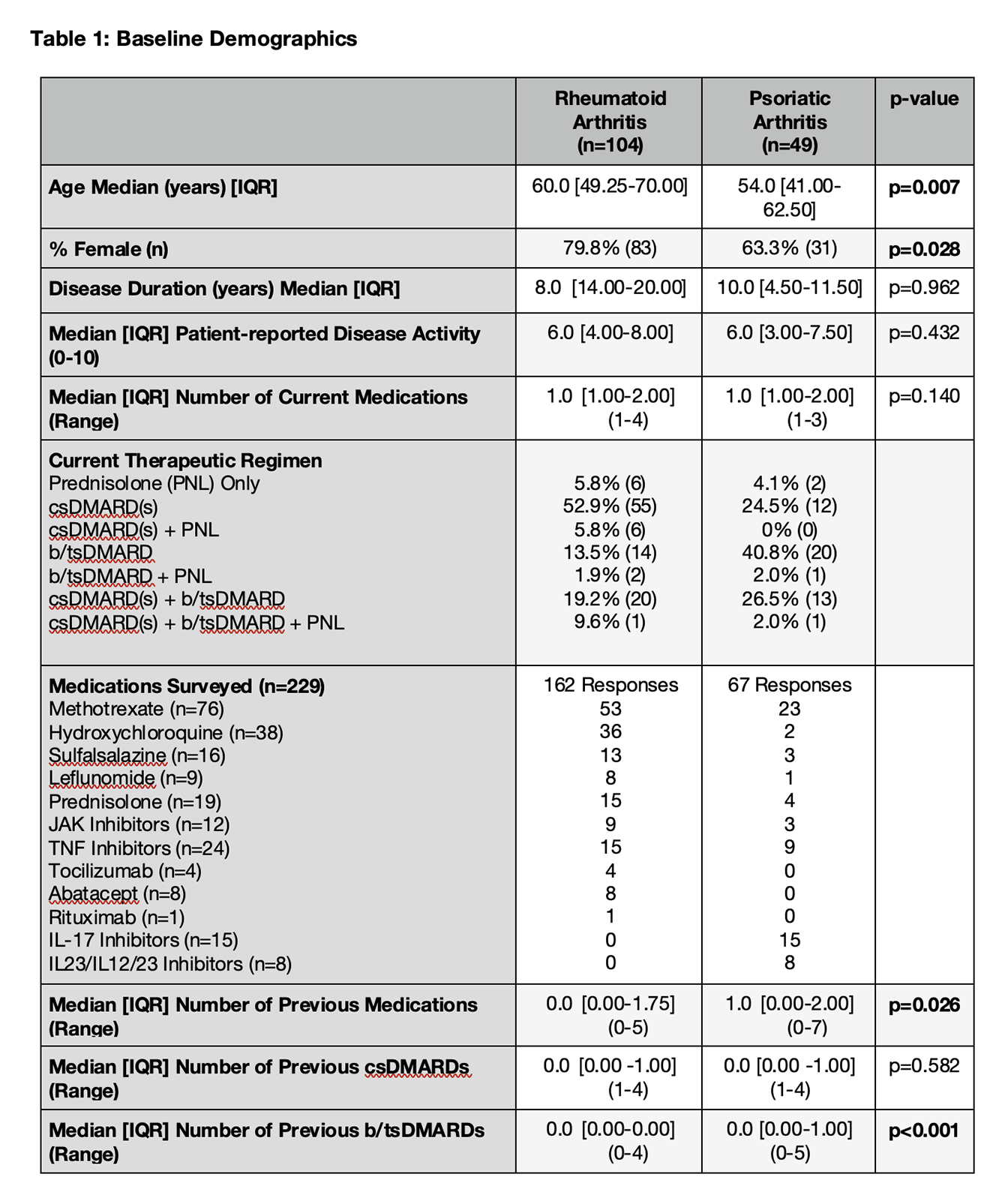
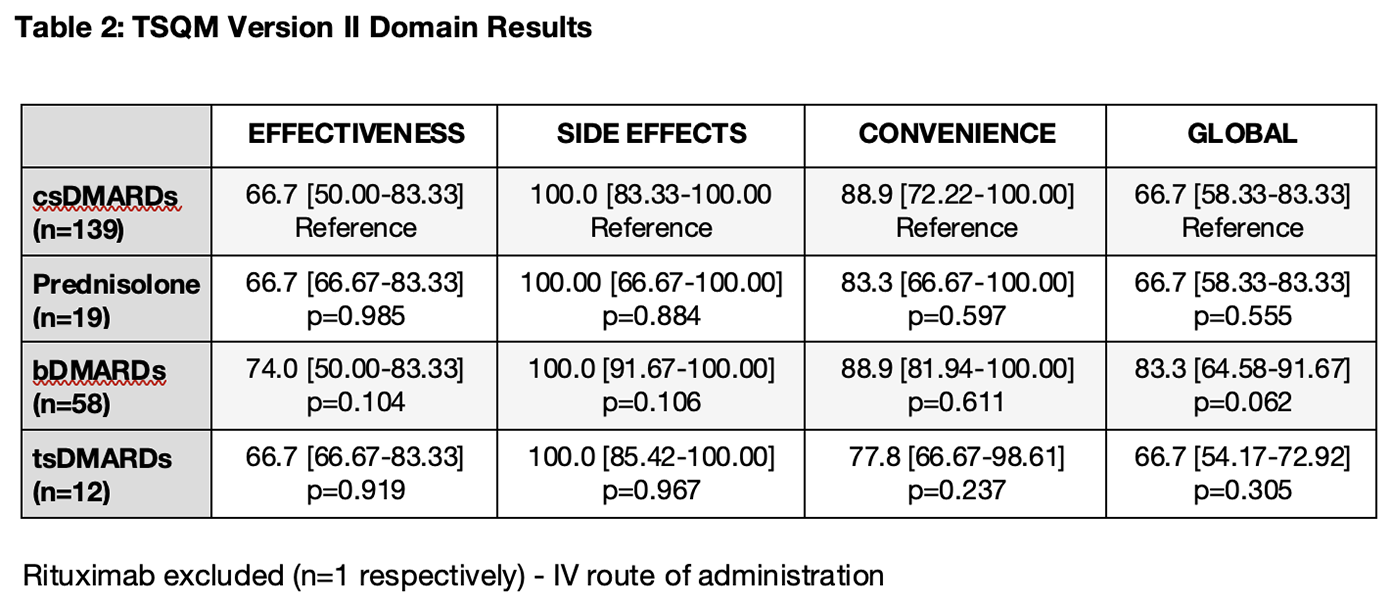
Results: 153 patients were recruited (104 patients with RA and 49 patients with PsA) and completed surveys for 229 medications (162 for RA and 67 for PsA). Patients with RA were older (60.0 vs. 54.0 years; p=0.007), more likely to be female (79.8% vs. 63.3%; p=0.028), and had less exposure to previous b/tsDMARDs (Table 1). A significantly higher proportion of surveyed patients with PsA were on current b/tsDMARDs compared to patients with RA (71.4% vs. 35.6%; p<0.0001). There were no significant differences in the patient treatment satisfaction scores for patients with RA vs. PsA across all domains: Effectiveness (66.7 vs. 75.0; p=0.176), Side Effects (100.0 vs. 100.0; p=0.429), Convenience (88.9 vs. 88.9; p=0.639) and Global (66.7 vs. 75.0; p=0.353). The only baseline variable correlating with treatment satisfaction was patient-reported disease activity, which was moderately associated with the Effectiveness (rho=-0.427; p<0.001) and Global Satisfaction (rho=-0.383; p<0.001) domains. A majority of patients (61.6%) reported no side effects associated with their medications. The proportion of patients reporting dissatisfaction due to side effects interfering with their “physical health and ability to function”, “mental function” and “mood or emotions” were 28.7%, 14.3% and 11.3% respectively. With csDMARDs serving as the reference group, Global Satisfaction was highest with bDMARDs (83.3 vs. 66.7; p=0.062), but similar in patients on tsDMARDs (66.7; p=0.305) and Prednisolone (66.7; p=0.555) (Table 2).
Conclusion: In this single-center cross-sectional observational study, treatment satisfaction with medications was similar among patients with RA and PsA, and was significantly driven by patient-reported disease activity. A minority of patients reported dissatisfaction with medication side effects, with the main concern being the impact on physical health and function. Further analysis in a large prospective cohort of patients commencing medications is needed to assess differences between therapeutic classes and modalities
REFERENCES:
Atkinson MJ, Kumar R, Cappelleri JC, et al. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health 2005;8 Suppl 1:S9-S24. Those seeking information regarding or permission to use the TSQM are directed to IQVIA at
Acknowledgements: NIL.
Disclosure of Interests: Shruthikah Satheesan: None declared, Kate Findeisen: None declared, Raychel Barallon: None declared, William Tillett Abbvie, Amgen, Celgene, Eli-Lilly, Janssen, Novartis, Pfizer, UCB, Abbvie, Amgen, Celgene, Eli-Lilly, Janssen, Novartis, Pfizer, UCB, Abbvie, Amgen, Celgene, Eli-Lilly, Janssen, Novartis, UCB, Anna Antony Eli Lilly, UCB.