

Background: Guselkumab (GUS) has demonstrated significant efficacy across disease domains in controlled clinical trials of patients with active psoriatic arthritis (PsA). [1,2] Recent CorEvitas PsA/Spondyloarthritis (SpA) Registry analyses confirmed real-world GUS effectiveness, showing that patients with persistent on-label GUS use at 6 months had significant mean improvements from baseline in peripheral joint and skin symptoms and patient-reported pain. [3]
Objectives: Here we reported secondary findings from the CorEvitas PsA/SpA Registry to further assess changes in patient-reported outcomes (PROs) among patients with persistent GUS use from baseline through the 6-month visit (On-Label GUS Persisters).
Methods: This analysis included registry patients who initiated on-label GUS after FDA approval for active PsA (7/13/2020; 100 mg subcutaneous at Weeks 0 & 4 then every 8 weeks) and were On-Label GUS Persisters. Demographics, PsA disease activity, PROs, and medication history at GUS initiation (baseline visit) were summarized descriptively. Among On-Label GUS Persisters not meeting response criteria at baseline, response rates at 6 months were determined for established outcomes related to improvements or achievement of low levels of disease activity in patient-reported pain (0-100 mm visual analog scale [VAS]), patient global assessment of arthritis + psoriasis (PtGA; 0-100 mm VAS), and Health Assessment Questionnaire-Disability Index (HAQ-DI; 0-3). Unadjusted, nominal p-values were calculated using a single-proportion, one-sided test to determine if response at the 6-month visit differed from 0% at baseline. Mean (95% CI) change from baseline to 6 months was determined for fatigue (0-100 mm VAS); nominal p-value calculated using a paired t-test at α=0.05.
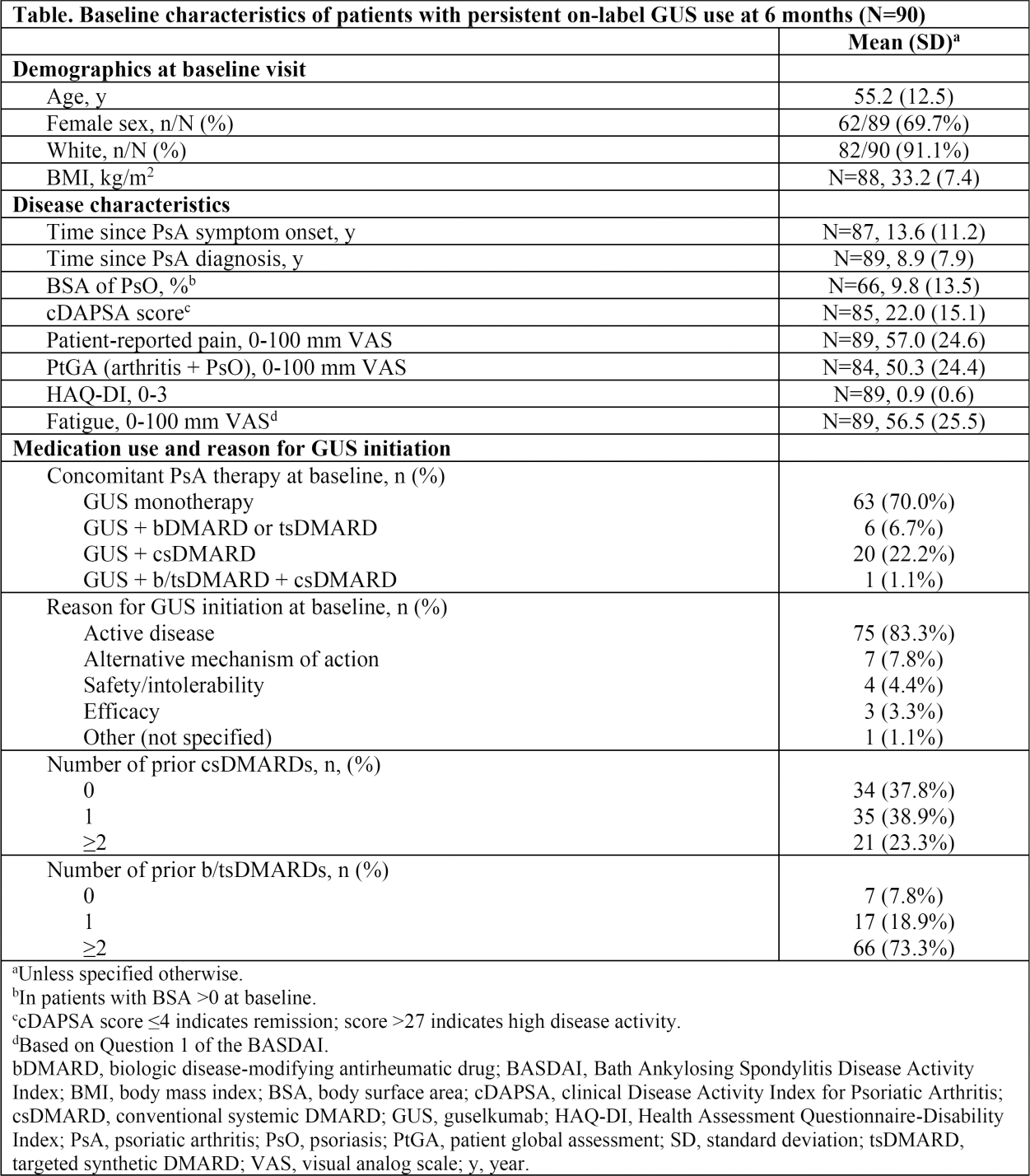
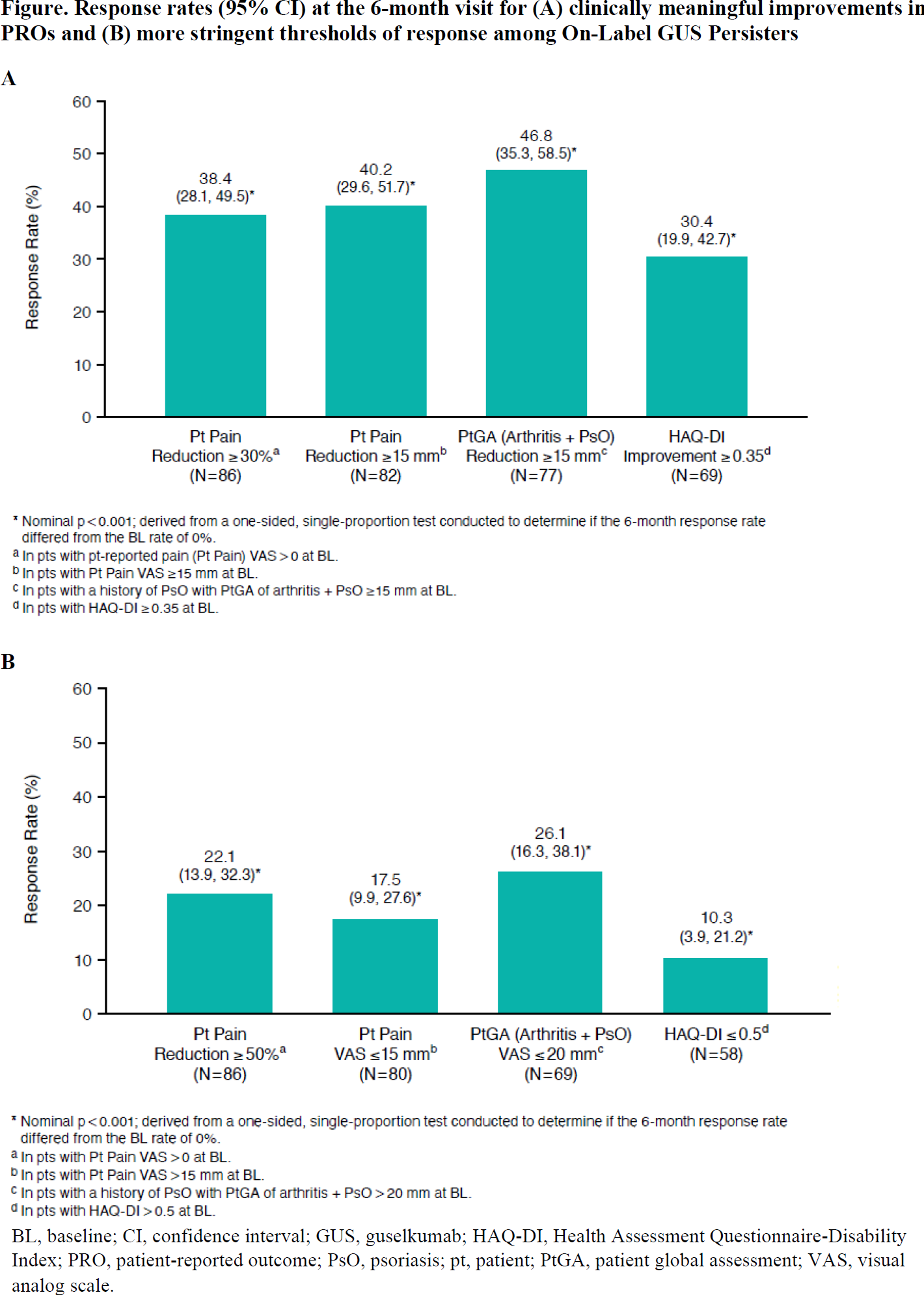
Results: Among 114 on-label GUS initiators with a 6-month follow-up visit, 90 (79%) had persistent on-label GUS use. On average, patients had longstanding, treatment-resistant, active PsA ( Table 1 ). Baseline patient-reported pain, HAQ-DI, and fatigue scores were 57.0, 0.9, and 56.5, respectively. Substantial proportions of On-Label GUS Persisters experienced clinically meaningful improvements in pain (38% with ≥30% reduction and 40% with ≥15-mm reduction), overall joint and skin disease (47% with ≥15-mm reduction in PtGA), and physical function (30% with HAQ-DI improvement ≥0.35; all nominal p<0.001; Figure 1 ). Further, up to one quarter of patients achieved the more stringent thresholds of response, generally representing a major response or minimal disease activity, including 22% with ≥50% reduction in pain, 18% with pain score ≤15 mm, 26% with PtGA score ≤20 mm, and 10% with HAQ-DI ≤0.5 (all nominal p<0.001; Figure 1 ). Mean change (95% CI) in fatigue from baseline at 6 months was -8.8 (-14.9, -2.7; nominal p=0.005).
Conclusion: In this real-world population of patients with treatment-resistant active PsA and persistent on-label GUS use, consistent with prior results for physician-reported endpoints of joint and skin disease, patients reported meaningful improvements in pain, physical function, and fatigue. These represent difficult-to-treat domains that are important contributors to the health-related quality of life of patients.
REFERENCES: [1] Deodhar. Lancet 2020;395:1115-25.
[2] Mease. Lancet 2020;395:1126-36.
[3] Mease. 6-M Persistence and Multi-Domain Effectiveness of GUS in Adults with PsA: RW Data from the CorEvitas Registry [abstr]. Maui Derm ; June 21-24, 2023; Colorado Springs, CO.
Acknowledgements: NIL.
Disclosure of Interests: Natalie J. Shiff Owns or has owned within the past 3 years stock in AbbVie, Gilead, Iovance, Jazz Pharmaceuticals, Johnson & Johnson, Novo-Nordisk, Novavax, Pfizer, and Viatris, Employee of Janssen Scientific Affairs, LLC, Salary support from the Childhood Arthritis and Rheumatology Research Alliance within the past 3 years, Philip J. Mease Speaker bureau support from AbbVie, Acelyrin, Aclaris, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Inmagene, Janssen, Moonlake Pharma, Novartis, Pfizer, SUN Pharma, UCB, Ventyx, and Xinthera, Consulting fees from AbbVie, Acelyrin, Aclaris, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Inmagene, Janssen, Moonlake Pharma, Novartis, Pfizer, SUN Pharma, UCB, Ventyx, and Xinthera, Research support from AbbVie, Acelyrin, Aclaris, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Inmagene, Janssen, Moonlake Pharma, Novartis, Pfizer, SUN Pharma, UCB, Ventyx, and Xinthera, Alexis Ogdie Consultant fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, CorEvitas, Eli Lilly, Gilead, Happify Health, Janssen, Novartis, Pfizer, and UCB, Grant/research support to the University of Pennsylvania from AbbVie, Janssen, Novartis, and Pfizer and to Forward from Amgen; her husband has received royalties from Novartis, John Tesser Speaker bureaus for AbbVie, Amgen, Aurinia, AstraZeneca, Bristol Myers Squibb (through 2021), GlaxoSmithKline, Janssen, Lilly, Pfizer, and Sanofi/Genzyme, Advisory boards/consultant for AbbVie, Amgen, AstraZeneca, Aurinia, Bristol Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Lilly, Pfizer, Novartis, Samumed/Biosplice, Sanofi-Genzyme, and UCB, Research grants and support from AbbVie, Alpine, Amgen, Anthrosi Therapeutics, Bendcare, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, CSL Behring, Corevitas, DRL, Emerald, Exagen, Genentech, Gilead, Global Health Living Foundation, Horizon, Janssen, Kolon TissueGene, Lilly, Mitsubishi, Organogenesis, Pfizer, Samumed/Biosplice, Selecta, Setpoint, Sun Pharma, Takeda, and Vorso, Iris Lin Own stock or stock options in Johnson & Johnson, Employee of Janssen Scientific Affairs, LLC, Soumya D. Chakravarty own stock or stock options in Johnson & Johnson, Employee of Janssen Scientific Affairs, LLC, Michael Kelleman Employee of CorEvitas, LLC, Rhiannon Dodge Employee of CorEvitas, LLC, Robert R. McLean Employee of CorEvitas, LLC, Aaron Broadwell Speaker fees from AbbVie, Amgen, Eli Lilly, Horizon, Janssen, Mallinckrodt, Novartis, Pfizer, Radius, Sanofi/Regeneron, and UCB, Consulting fees from AbbVie, Amgen, Aurinia, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, and Sandoz, Arthur Kavanaugh Consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Janssen, Eli Lilly, Merck, Novartis, Pfizer, and UCB, Joseph F. Merola Consulting fees and investigator honoraria from AbbVie, Amgen, Biogen, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Janssen, Leo Pharma, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB.