

Background: Core outcome sets (COS) represent the minimum set of domains and the measurement instruments that should be reported in every trial and longitudinal study of a specific condition. The existing Rheumatoid Arthritis (RA)-COS comprises eight outcome domains: tender joints, swollen joints, pain, physician global assessment, physical disability, patient global assessment, acute phase reactants and fatigue. A composite outcome (index) is often chosen as primary trial outcome as it offers an appealing approach to boost statistical power and lower the risk of type-1 errors stemming from multiple individual statistical tests for each core domain. However, such composites may obscure instances where treatment effects are dissimilar between domains.
Objectives: To explore the associations of results on separate core domains with the American College of Rheumatology (ACR) 20% response in trials assessing the effect of targeted interventions in RA.
Methods: A meta-epidemiological study on RA patients included in randomised trials investigating biologics and targeted agents compared to placebo or conventional disease modifying antirheumatic drugs was performed. A meta-regression analysis associated each trial result expressed as odds ratio for the ACR 20% response (experimental vs comparator treatment) with the result of each of the RA–COS domains expressed as standardized mean difference.
Results: 115 trials involving 55,422 RA patients were eligible. The odds ratio for achieving ACR 20% response was 3.19 (95%CI: 2.96 to 3.44) for the experimental interventions relative to the comparators. The median number of COS domains reported was 6; 18 trials reported only 1 domain and 17 reported all 8. Univariable meta-regression analyses indicated that each of the eight core domains was significantly associated with ACR 20% response, but the association with improvements in physical disability was strongest. In the 17 studies that reported all eight core domains, the association with improvements in fatigue was strongest.
Conclusion: In this meta-epidemiological study of RA trials, the association of individual components with the ACR 20% response was strongly influenced by both the amount and characteristics of missing data on those components, precluding strong conclusions. Our data suggest that fatigue could be an important contributor to the ACR 20% response, but this is based on limited data. Despite recommendations, most trials do not cover all eight core domains. Reviewers and journal editors should make a better effort and insist that trialists adhere to current guidelines.
REFERENCES: [1] Jamie Kirkham, Robin Christensen, Maarten Boers. Use of composite outcomes facilitate core outcome set uptake in rheumatoid arthritis trials. Ann Rheum Dis. 2020 Feb;79(2):301-302. doi: 10.1136/annrheumdis-2019-216256.
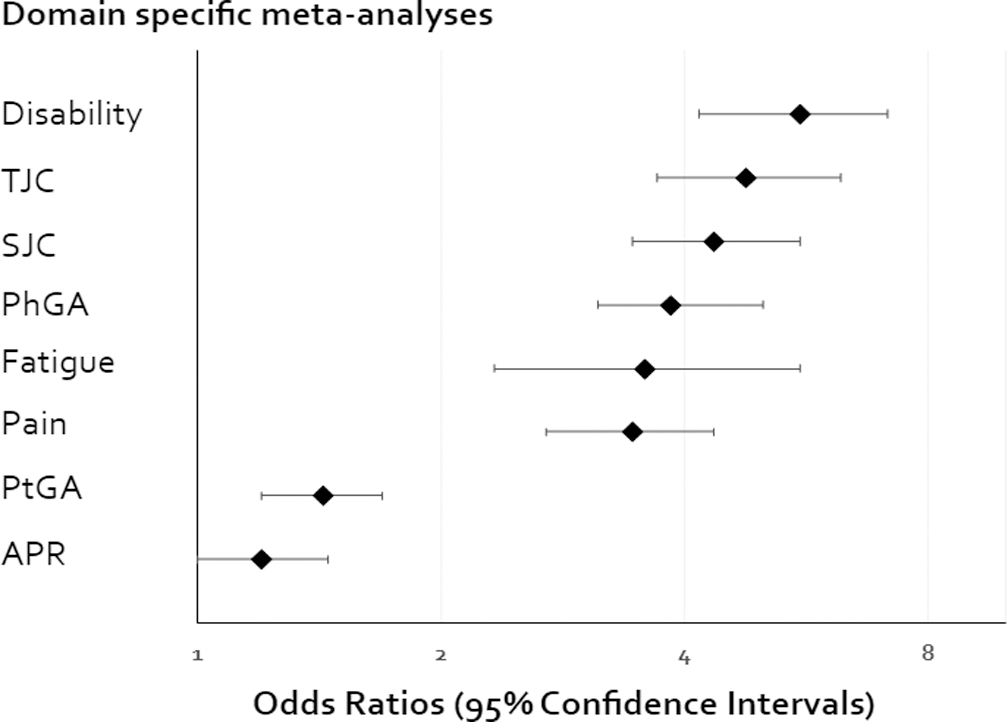
| Regression analyses based on all included studies | |||
|---|---|---|---|
| Core domain | Comparisons | OR | 95%CI |
| Disability | 236 | 5.56 | (4.17 to 7.14) |
| TJC | 183 | 4.76 | (3.70 to 6.25) |
| SJC | 183 | 4.35 | (3.45 to 5.56) |
| PhGA | 153 | 3.85 | (3.13 to 5.00) |
| Fatigue | 73 | 3.57 | (2.33 to 5.56) |
| Pain | 186 | 3.45 | (2.70 to 4.35) |
| PtGA | 187 | 1.43 | (1.20 to 1.69) |
| APR | 182 | 1.20 | (1.00 to 1.45) |
| Regression analyses based on studies with complete COS-reporting only | |||
|---|---|---|---|
| Core domain | Comparisons | OR | 95%CI |
| Fatigue | 30 | 3.39 | (1.80 to 4.26) |
| Disability | 30 | 3.35 | (1.88 to 5.99) |
| PhGA | 30 | 3.32 | (2.13 to 5.18) |
| SJC | 30 | 2.99 | (1.84 to 4.88) |
| TJC | 30 | 2.77 | (1.80 to 4.26) |
| PtGA | 30 | 2.21 | (1.46 to 3.33) |
| APR | 30 | 2.14 | (1.24 to 3.68) |
| Pain | 30 | 1.86 | (1.34 to 2.58) |
TJC : Tender Joint count; SJC : Swollen joint count; PhGA : Physician global assessment; PtGA : Patients global assessment; APR : Acute phase reactant. CI: Confidence interval. OR: Odds Ratio. Meta-regression was done on log odds scale, but for ease of interpretation, the back transformed odds ratio is reported.
Based on odds ratio the core domains can be categorised into two according to their association to the ACR20 response.
TJC : Tender Joint count; SJC : Swollen joint count; PhGA : Physician global assessment; PtGA : Patients global assessment; APR : Acute phase reactant. CI: Confidence interval. OR: Odds Ratio
Acknowledgements: NIL.
Disclosure of Interests: None declared.