

Background: Juvenile Idiopathic Arthritis (JIA) represents the most prevalent inflammatory rheumatic condition in childhood. Its heterogeneous nature presents challenges in identifying feasible biomarkers for effective disease monitoring. Serum calprotectin (sCal) has emerged as a potentially valuable biomarker for precisely assessing inflammatory activity in JIA. Despite various commercially available methods for sCal determination, their usage remains limited and lacks validation.
Objectives: To assess the diagnostic accuracy, define an optimal threshold, and evaluate the association between disease activity status in patients with JIA and serum calprotectin measurements obtained through chemiluminescence (CLIA) and solid-phase enzyme immunoassay (EIA) techniques within a real-world clinical setting.
Methods: Serum samples from 25 pediatric patients with JIA were analyzed. sCal levels were determined by CLIA and EIA methodologies. Disease activity data was collected through JADAS-27 and the Anink ACR-modified criteria (1). We conducted a comparative analysis of results obtained through CLIA and EIA techniques. Additionally, we assessed the association between distinct biomarkers and disease activity.
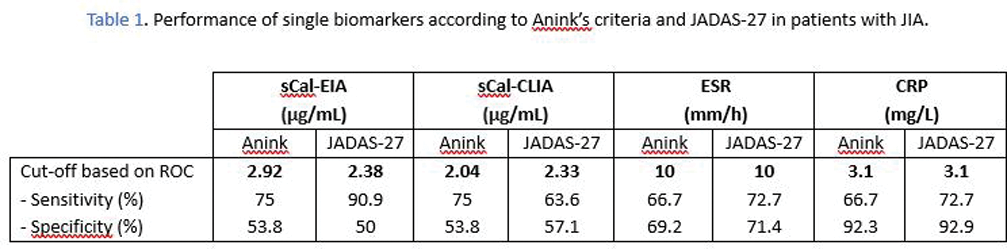
Results: The tests demonstrated robust performance of sCal. The ideal cut-off value for sCal in identifying disease activity, as determined by ROC analysis related to JADAS-27, was 2.3 µg/mL for both CLIA and EIA techniques. Remarkably, this value aligned with the threshold routinely utilized in our center’s daily clinical practice. When evaluating disease activity based on Anink’s criteria, the identified optimal thresholds were 2.0 µg/mL for sCal CLIA and 2.9 µg/mL for sCal EIA. Notably, the sCal CLIA identified threshold (2.0 µg/mL) coincided with the one recommended by its commercial kit. In contrast, the thresholds proposed for CRP and ESR (3.1 mg/L and 10 mm/h, respectively), according to both JADAS-27 and Anink, were lower than the values routinely employed in daily clinical practice (5 mg/L for CRP and 20 mm/h for ESR).
A good correlation was observed between sCal levels obtained by CLIA and EIA Kendall’s tau-b 0.71, p<0.001).
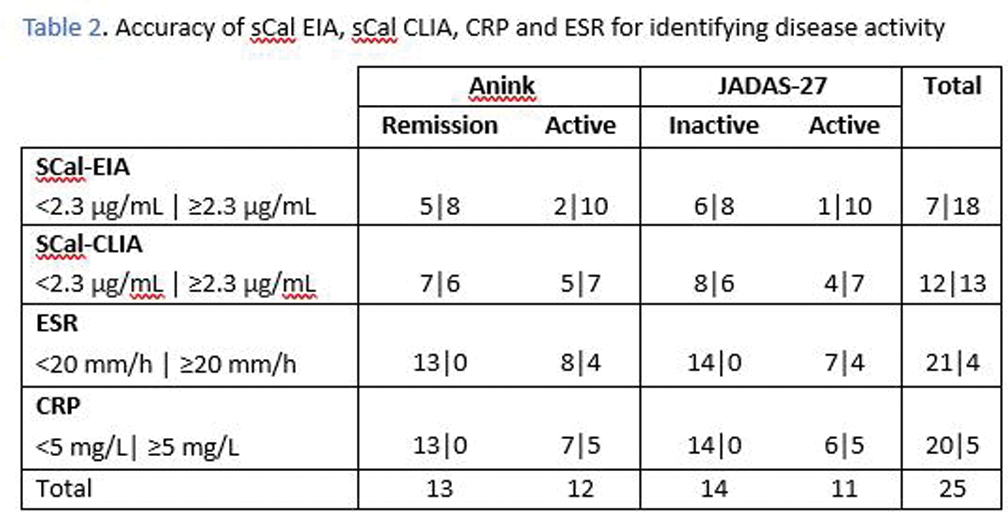
The sensitivity of sCal outperformed the conventional inflammatory biomarkers CRP and ESR, exhibiting superior accuracy in distinguishing patients with active disease. In our cohort, a notable case involved an active patient experiencing concurrent arthritis and uveitis during data collection, exhibiting a significant discrepancy in biomarker measurements showing elevated sCal levels (≥2.3µg/ml) but without a corresponding increase in CRP or ESR.
However, sCal showed reduced specificity compared to CRP and ESR, resulting in the conventional biomarkers proving more effective in identifying patients in a state of remission.
Conclusion: Serum calprotectin, determined through CLIA and EIA, emerges as a valuable biomarker for monitoring disease activity in patients with JIA. It exhibits good sensitivity for identifying active patients at its cutoff point of 2.3 µg/mL, especially through EIA.
The results suggest sCal is superior for identifying activity, while CRP and ESR excel in distinguishing remission.
The combined use of various serum biomarkers could enhance the precision of disease activity assessment in JIA.
REFERENCES: [1] Anink J, Van Suijlekom-Smit LW, Otten MH, Prince FH, van Rossum MA, Dolman KM, et al. MRP8/14 serum levels as a predictor of response to starting and stopping anti-TNF treatment in juvenile idiopathic arthritis. Arthritis research & therapy. 2015;17(1):200
Acknowledgements: NIL.
Disclosure of Interests: None declared.