

Background: Gout is the most common inflammatory arthritis caused by hyperuricemia. And the incidence of juvenile gout was rising worldwide. The specific mechanisms and treatment strategies remain unclear. Febuxostat is commonly used to lower blood uric acid levels in adult gout patients, but its efficacy in juvenile gout patients with different genetic backgrounds has not been adequately studied. Numerous prior studies have indicated that the T allele genotype at the C677T site of the MTHFR gene increases hyperuricemia risk by elevating plasma homocysteine levels. Similarly, the ABCG2 gene, implicated in gout susceptibility, affects uric acid levels through the regulation of renal excretion and reabsorption.
Objectives: This study aims to investigate the relationship between variations of MTHFR and ABCG2 gene and both the gout onset in children and the efficacy of Febuxostat.
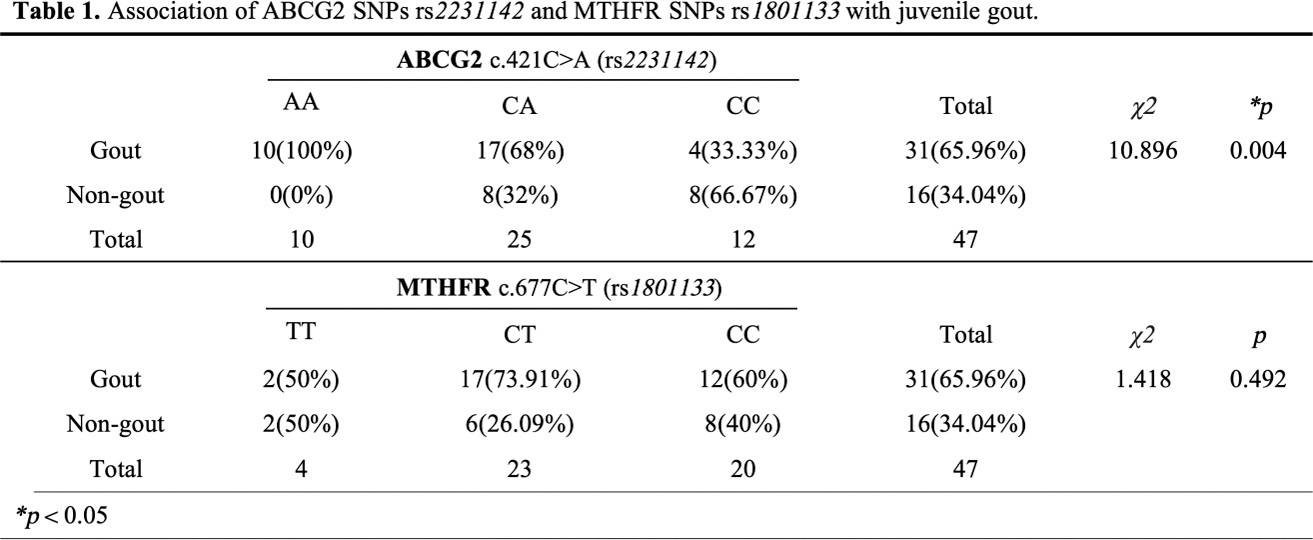
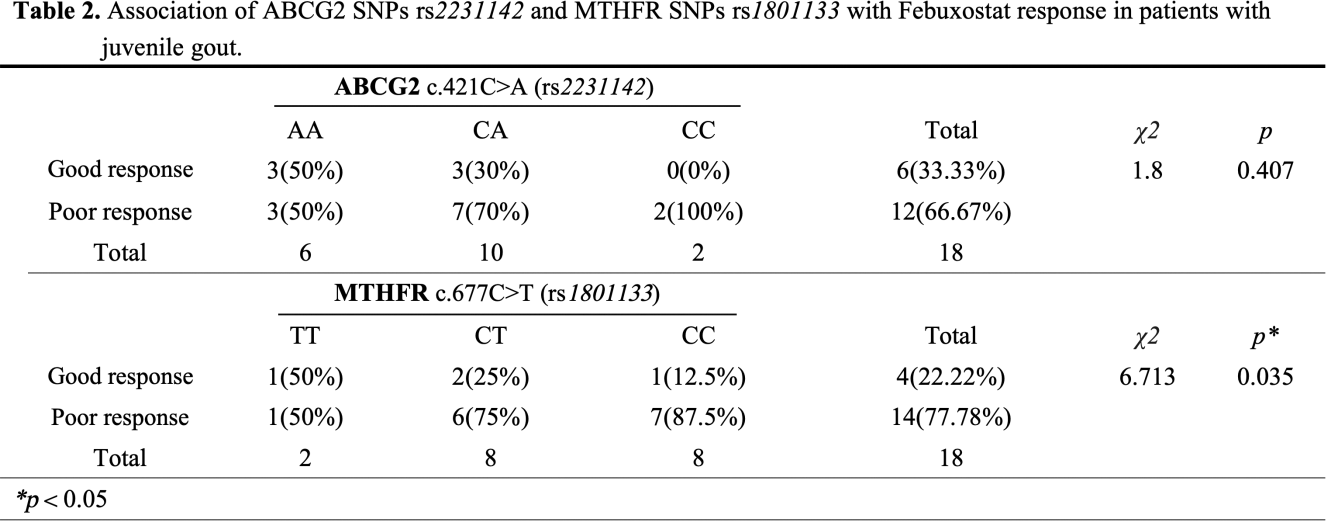
Methods: These studies were approved by theInstitutional Review Board of Guangdong Second provincial General Hospital.The study enrolled 31 pediatric gout patients, 18 of whom received Febuxostat treatment regularly(40mg per day) for 12 weeks, alongside 16 non-gout children (aged 10 to 18 years, male). EDTA whole blood samples were collected for genetic testing of the ABCG2 gene c.421C>A (rs 2231142 ) and the MTHFR gene c.677C>T (rs 1801133 ). Participants were categorized based on these gene sites into three groups: homozygous defect (AA), heterozygous defect (CA), and full-function (CC) for ABCG2; and similarly for MTHFR. Treatment efficacy was determined based on blood uric acid levels, with levels exceeding 420 μ mol/L classified as a “poor response” and levels at or below 420 μ mol/L as a “good response.” The Chi-square test was employed for statistical analysis.
Results: Significant differences were observed in ABCG2 (rs 2231142 ) between juvenile gout and non-gout group ( p =0.0043), but no correlation was noted in the response to Febuxostat treatment (Table 1 ). The juvenile gout group contained 10 cases in the homozygous defect group (AA), 17 in the heterozygous defect group (CA), and 4 in the full-function group (CC). In contrast, the non-gout group had 0, 8, and 8 cases, respectively. A statistically significant difference was found in MTHFR (rs 1801133 ) between groups with varying responses to Febuxostat treatment ( p =0.035), with a higher proportion of CT and CC genotypes in the good response group (Table 2 ). However, no statistical difference was noted in ABCG2 (A) rs 2231142 between the two Febuxostat treatment groups.
Conclusion: Our results showed that ABCG2 genotyping played an important role in the risk of gout in juvenile. When predicting or evaluating the efficacy of febuxostat, MTHFR genotyping may have a certain guiding role in the selection of research plans.
REFERENCES: [1] Ciferska, H, Pavelcova, K, Vachek, J, et al. POS0354 DETECTION OF ABCG2 VARIANTS IN ENCODING OF uric acid TRANSPORTERS ASSOCIATED WITH THE HYPERURICEMIA IN HAEMODIALYSIS PATIENTS ANN RHEUM DIS. 2021; 80 (Suppl 1): 407.2-408. doi: 10.1136/annrheumdis-2021-eular.2084.
[2] van der Pol, KH, Nijenhuis, M, Soree, B, et al. Dutch pharmacogenetics working group guideline for the gene-drug interaction of ABCG2, HLA-B and Allopurinol, and MTHFR, folic acid and methotrexate. EUR J HUM GENET. 2022; doi: 10.1038/s41431-022-01180-0.
Acknowledgements: This study was supported by Medical Scientific Research Foundation of Guangdong Province(B2022162, A2023045).
Disclosure of Interests: None declared.