

Background: Autoantibody production by long-lived plasma cells (PCs) have been implicated in the pathogenesis of systemic lupus erythematosus (SLE) but targeting them remains a therapeutic challenge.
Objectives: We aimed to investigate the safety and efficacy of the anti-CD38 monoclonal antibody daratumumab in moderate to severe SLE. This reagent depletes PCs and is approved for the treatment of multiple myeloma.
Methods: A single-center, phase 2, open-label investigator-initiated study was conducted in 10 patients with SLE receiving background standard therapy. Eligible patients were adults meeting the 2019 EULAR/ACR classification criteria, had a baseline SLE Disease Activity Index 2000 (SLEDAI-2K) score of ≥6, were positive for anti-double-stranded (ds)DNA antibodies and had failed or did not tolerate at least 2 previous state-of-the-art immunosuppressive drugs. Patients received 8 subcutaneous injections once weekly of 1800mg daratumumab and were followed-up for 36 weeks. Primary endpoint was the reduction of anti-dsDNA antibody levels at week 12. Secondary endpoints included safety and tolerability, reductions in Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) and Clinical Disease Activity Index (CDAI), SRI-4 responses, pharmacokinetics and immunological changes. This trial was supported by Janssen-Cilag and registered at
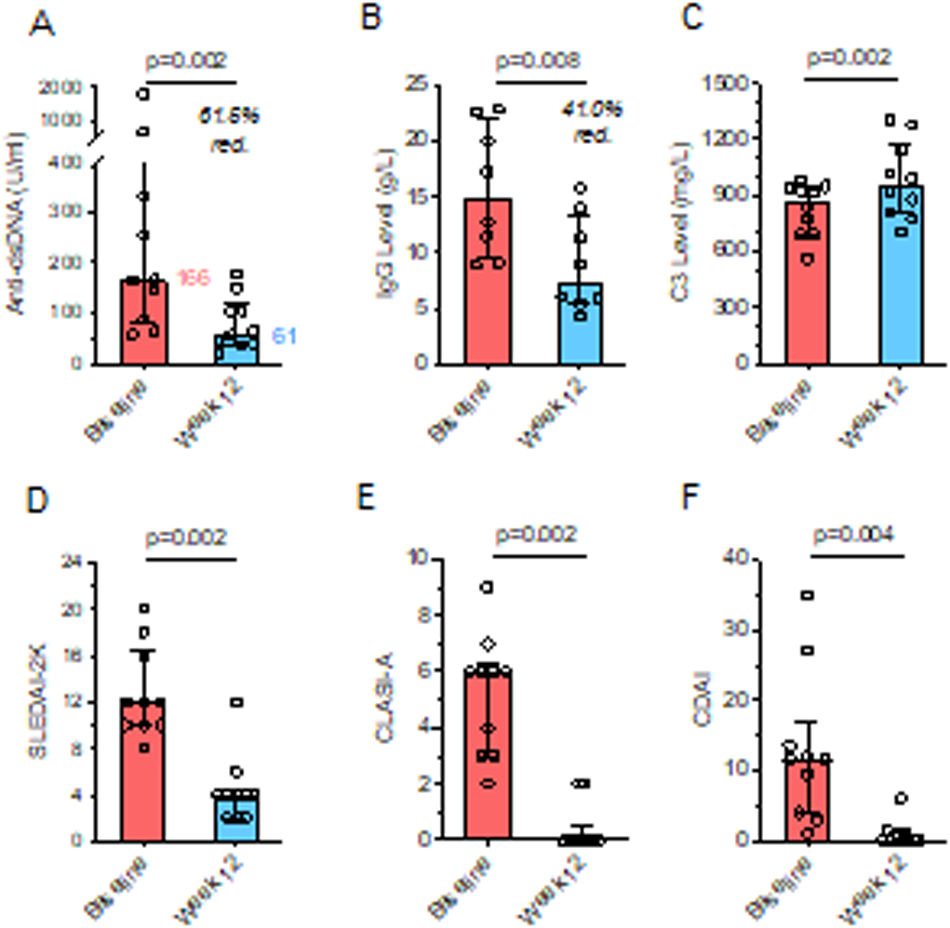
Results: Between August 2021 and January 2023, 10 patients were enrolled and received at least six doses of daratumumab. Anti-dsDNA antibodies decreased from a median 166.3 at baseline to 61.1 U/ml at week 12 (p=0.002), accompanied by significant clinical improvements, reflected in median reductions of SLEDAI-2K from 12 to 4 (p=0.002), CLASI-A from 6 to 0 (p=0.002) and CDAI from 11.5 to 0 (p=0.004), resulting in an SRI-4 response of 100%. Serum IgG levels decreased from 12.1 to 6.9 g/L, and serum complement C3 increased from 875 to 955 mg/L (p=0.002). During follow-up, two patients developed flares at week 16 and 24, respectively. At the final visit, SRI-4 response rate was 70%, despite reductions of daily prednisolone dosages from 6.25 at baseline to 5.0mg (p=0.016). No severe adverse events (SAEs) occurred. Treatment emergent AEs were mild-moderate, including hypogammaglobulinemia (5/10), nausea (4/10), headache (4/10), injection site reactions (3/10), COVID-19 (3/10) and herpes zoster (2/10). Pharmacodynamic analysis showed transient reductions of natural killer cells and plasmacytoid dendritic cells, while peripheral blood B and T cell numbers remained stable.
Conclusion: Daratumumab induced a therapeutically relevant reduction of pathogenic anti-dsDNA antibodies in SLE with a favorable safety profile. Clinical responses were rapid and durable, even in refractory cases, with efficacy in all major organ sites. These data justify the further development of CD38-targeting antibodies in the treatment of moderate-severe SLE.
Clinical and serologic responses of 8 weekly daratumumab injections in 10 SLE patients. Change from baseline of A) anti-dsDNA antibodies, B) serum IgG levels, C) complement factor C3, D) SLEDAI-2K, E) CLASI-A and F) CDAI score.
REFERENCES: NIL.
Acknowledgements: This study was supported by Janssen-Cilag.
Disclosure of Interests: Tobias Alexander This study was supported by Janssen-Cilag, Lennard Ostendorf: None declared, Jan Zernicke: None declared, Jens Klotsche: None declared, Udo Schneider: None declared, Robert Biesen: None declared, Robin Kempkens: None declared, Qingyu Cheng: None declared, Laleh Khodadadi: None declared, Frederik Heinrich: None declared, Pawel Durek: None declared, Gerd R. Burmester: None declared, Mir-Farzin Mashreghi: None declared, Gerhard Krönke: None declared, Falk Hiepe: None declared.