

Background: informing residual risk to improve cardiovascular risk stratification is a major unmet need in rheumatoid arthritis (RA). Autoimmune phenomena and immune dysregulation, including humoral responses, are thought to play a role in risk excess. However, exact mediators are unknown. Age-associated B cells (ABCs) have emerged as multi-faceted pro-inflammatory mediators, also in the atherosclerosis microenvironment. Despite being expanded in autoimmunity, their role is ill-defined.
Objectives: to evaluate ABCs levels in the earliest stages of inflammatory arthritis and their potential role as biomarkers of atherosclerosis.
Methods: ABCs (CD19+CD21-CD11c+) were quantified by flow cytometry in Peripheral Blood Mononuclear Cells (PBMCs) samples from 58 early RA patients (2010 EULAR/ACR classification criteria; 69% RF, 66% ACPA; DAS28 5.28±1.13), 11 individuals with clinical-suspect arthralgia (CSA) (EULAR definition; 54% RF, 45% ACPA) and 33 healthy controls (HC). All patients were untreated at recruitment. Atherosclerosis occurrence was measured by Doppler-ultrasound. Cardiometabolic-related proteins were evaluated using high-throughput targeted proteomics.
Results: ABCs frequency within the CD19+ compartment was increased in RA patients compared with HC (2.96±2.47 vs 1.56±1.36 %, p=0.013). Equivalent results were observed within the total PBMC pool (p<0.001). CSA individuals showed similar ABCs levels than RA (p=0.495). No associations with disease activity, symptom duration or RF/ACPA positivity were retrieved (all p>0.050). Higher ABCs counts at onset were linked to poor/moderate response to DMARDs (EULAR criteria) at 6 months compared to patients presenting a good response (p=0.022). ABCs frequency was positively correlated with serum levels of proinflammatory cytokines (IL6, IFNg and TNF, all p<0.050) in RA. Proteomic analyses revealed that ABCs were associated with 11 proteins (protein-protein interaction: p=3.3·10 -10 ) (Figure 1A) related to B-cell activation, T-cell-dependent B-cell activation and macrophages/foam cell activation (Figure 1B). ABCs numbers were associated with atherosclerosis occurrence (p=0.006) and correlated with the number of atheroma plaques (r=0.346, p=0.010) in RA patients, whereas no correlation between cIMT and ABCs was found (p=0.322). ABCs counts were associated with atherosclerosis occurrence in univariate models (OR [95% CI], p: 1.260 [1.051-1.510], p=0.012), and remained as independent predictors of atherosclerosis after adjusting for traditional risk factors (OR [95% CI], p: 1.284 [1.037-1.589], p=0.022). Moreover, adding ABCs levels to the mSCORE improved risk stratification over the mSCORE alone based on diagnostic/classification statistics and net reclassification improvement (Figure 1C).
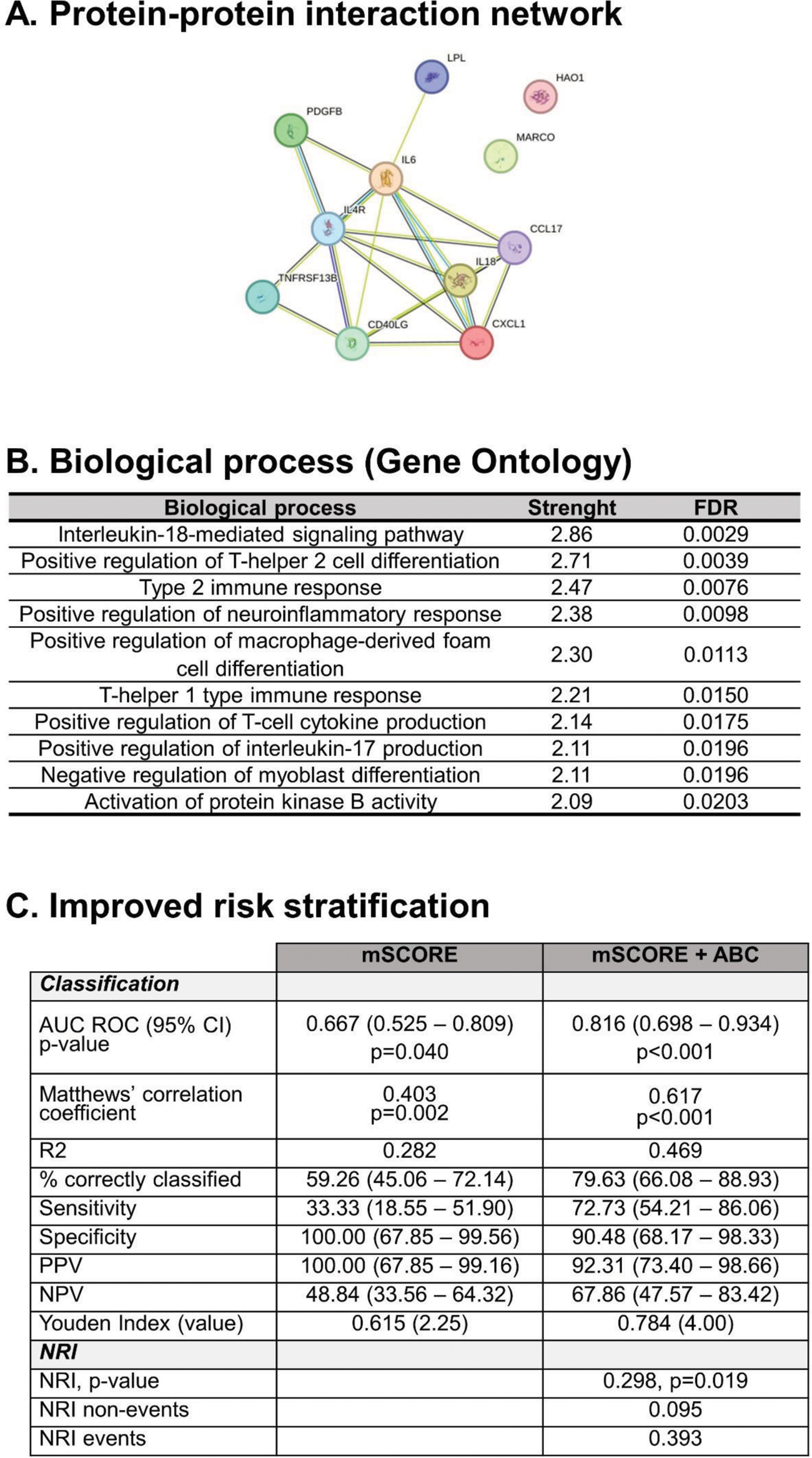
Conclusion: ABCs expansion may be an early biomarker of atherosclerosis in arthritis, with incremental value in improving risk stratification over existing algorithms. ABCs may be a missing link between humoral responses and atherosclerosis in autoimmunity.
REFERENCES: NIL.
Acknowledgements: ISCIII (PI21/00054 and FI22/00148 grants).
Disclosure of Interests: None declared.