

Background: Patients with rheumatoid arthritis (RA) display different trajectories in the improvement of disease states. Discerning how RA evolves, how it differs between patients and which factors influence these trajectories, is relevant to identify opportunities to improve disease management. Previous studies identified smooth time trajectories of rapid, slow or no progression of DAS28. In real life, we observe more chaotic disease evolvements and particularly the detours could indicate a lack of adequate treatment.
Objectives: Discern trajectories of disease states of early RA over 1.5 years from the first outpatient clinic visit using pseudo-time graphs-based analyses.
Methods: We collected visits of early RA patients over 1.5 years from the LUMC outpatient clinic between 2011-2022 for all patients with at least two visits (n=1,237 patients, 5,017 visits). All visits captured swollen and tender joint counts (SJC, TJC), hemoglobin, thrombocytes, Mean Corpuscular Volume (MCV), leukocytes, and Erythrocyte Sedimentation Rate (ESR). Neither serology, sex, nor age were used during clustering. After normalization, we clustered visits using cosine similarity and the Leiden algorithm. Time of visit was excluded from the clustering. We identified the number and stability of clusters (defined as disease states) with Rand index and SVM predictions. We identified similar patient trajectories by Optimal Matching and the Leiden algorithm. To validate the findings, we applied the pipeline to the TACERA cohort consisting of 244 patients with early seropositive RA recruited from pre-July 2014 till Dec 2014 with a total of 636 visits with intervals of at least 6 months.
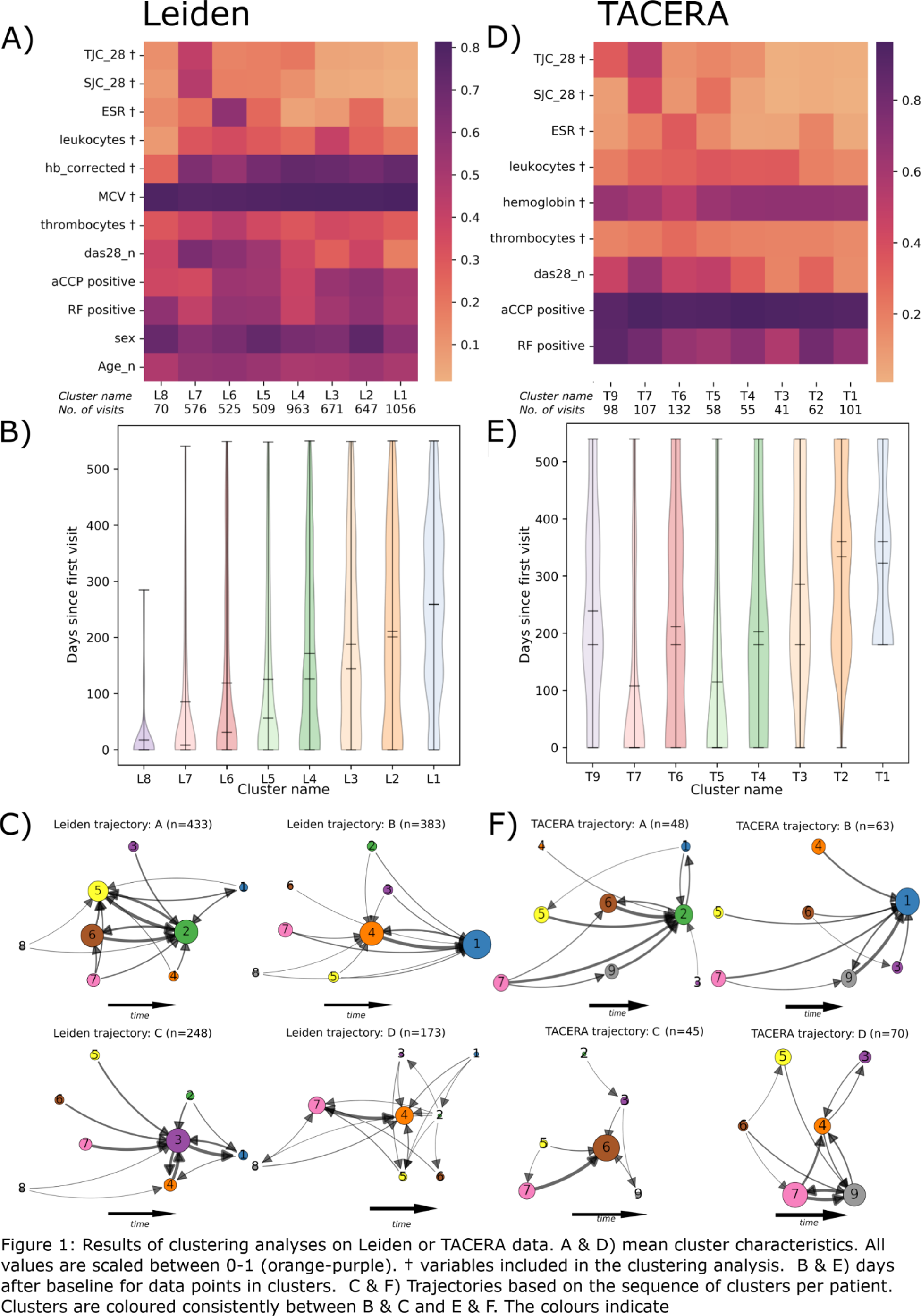
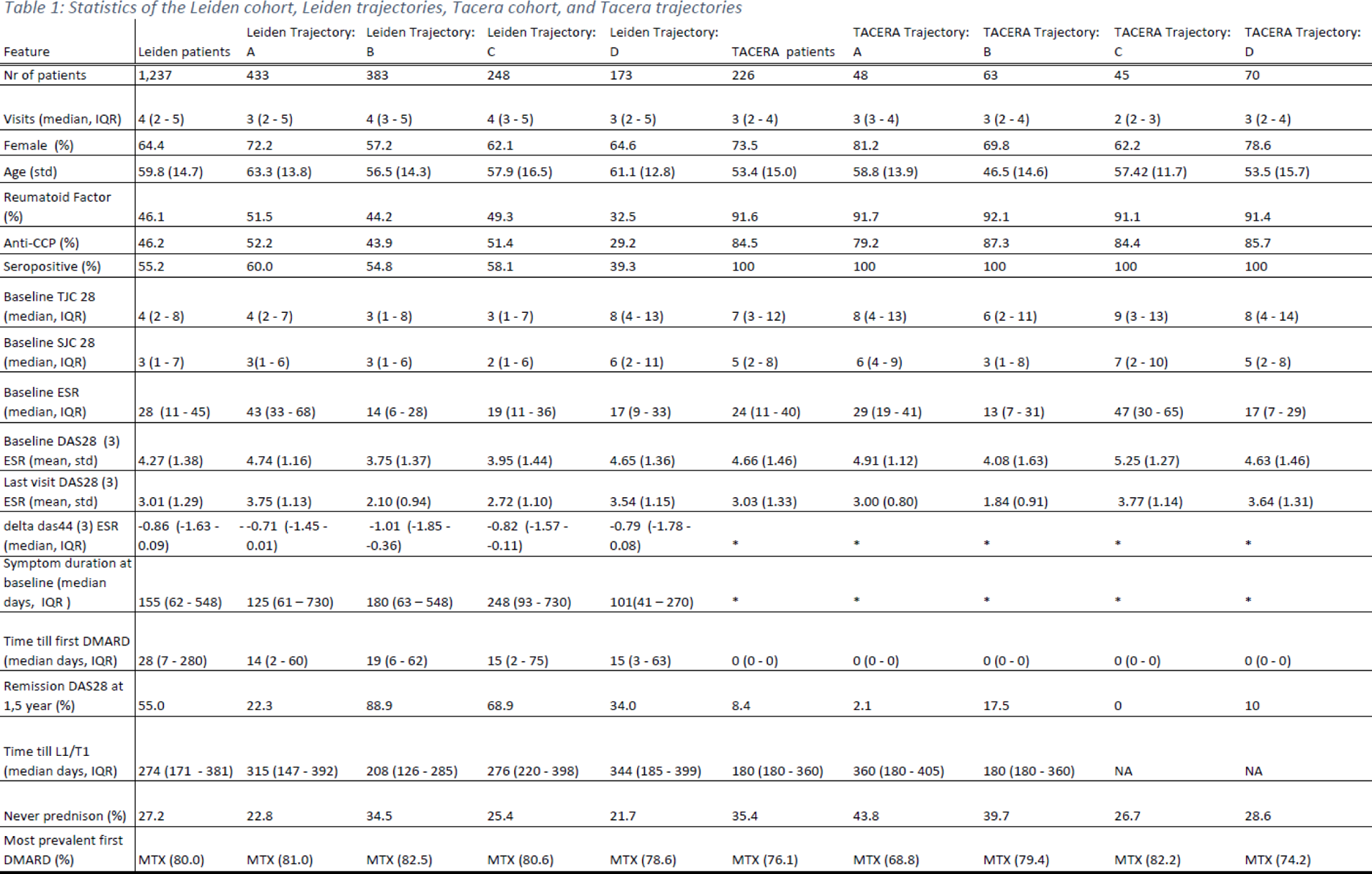
Results: The Leiden data showed eight disease states with SJC/TJC, ESR and leukocytes as the major discriminating factors (Figure 1A). One cluster (L1) was the clinically optimal disease state with the least inflammation overall. Though each cluster contained visits from baseline until the last visit (within 1.5 years), on average the low disease activity clusters were enriched for later visits (Figure 1B). The disease state sequences consisted of 4 distinct trajectories (Figure 1C): A - high ESR clusters at start and end (L6,5,2); B - rapid progression towards the ideal cluster (L1); C - transition through high leucocyte state L3; D - poor prognosis (L4,7). In the independent analysis on TACERA, we found similar clusters and trajectories. The only difference between TACERA and Leiden is T9 (Figure 1.D) and L8. The trajectories and clusters revealed interesting features, marking differences in age, gender, and serostatus, even though these variables were not included in the clustering (Table 1). The differences were not driven by inclusion date, follow-up duration, symptom duration or time till methotrexate initiation. The patients in Trajectory B and C appear similar at baseline, though C has a less favourable trajectory. The trajectories could not be predicted by baseline variables, except from trajectory A. This trajectory A is characterized by high ESR from start to end and seems to get stuck in the almost optimal L2 with elevated ESR. We observed a similar DAS44 improvement over time, demonstrating that our results were not driven by unmeasured inflammation in the feet.
Conclusion: With our graph-based pseudo-time analysis we identified and replicated 4 trajectories in early RA. These trajectories draw a more granular picture than previously described, showing patients stranding in suboptimal disease states. Inflammation in joints, blood, and poor response are the main discriminatory factors. Our approach provides insights into opportunities for improving care, such as more intensive treatment at an earlier stage. Our next step will be to characterize the immune profiles of the different disease states and discern the impact of treatment decisions.
REFERENCES: NIL.
Acknowledgements: This project has received funding from Horizon Europe programme under grant agreement no. 101095052 (SQUEEZE), no. 101080711 (SPIDERR), and no. 777357 (RTCure, MRC/ABPI Inflammation and Immunology Initiative Grant (MRC reference numbers: G1001516 and G1001518) Towards a Cure for Early Rheumatoid Arthritis (TACERA). We thank Samantha Jurado-Zapata and David Steeman for their help in extracting and processing the electronic health record data from Leiden University Medical Centre.
Disclosure of Interests: Nils Steinz: None declared, Tjardo D. Maarseveen: None declared, Andrew Cope: None declared, John Isaacs: None declared, Aaron Winkler No conflict of interest applies to this abstract, Tom Huizinga: None declared, Abraham Yann No conflict of interest applies to this abstract, Rachel Knevel: None declared.