

Background: SHR0302, a potent selective JAK1 inhibitor, has demonstrated efficacy and safety in treating patients (pts) with active RA in a phase 2 trial (NCT03254966).
Objectives: This multicenter, randomized, placebo-controlled, double-blind phase 3 study (NCT04333771) was conducted to further assess the efficacy and safety of SHR0302 in pts with moderate to severe active RA who had an inadequate response to csDMARDs.
Methods: Eligible pts, aged 18-75 years, diagnosed with RA according to the 2010 ACR/EULAR criteria, exhibited moderately to severely active disease, and had an inadequate response to csDMARDs, were randomized (1:1:1) to receive either a placebo, 4 mg, or 8 mg of SHR0302, administered orally once daily for 24 weeks, stratified by concomitant csDMARDs use at baseline (either methotrexate at a dose of ≥15 mg/week or leflunomide at 20 mg/day; methotrexate at <15 mg/week or leflunomide at <20 mg/day; or no usage of either methotrexate or leflunomide). Subsequently, pts who were initially assigned to the placebo group were switched to receive SHR0302 4 mg for an additional 28 weeks, while those originally assigned to SHR0302 continued with their initial dosage. The primary endpoint was the proportion of pts who achieved a 20% improvement in the American College of Rheumatology response criteria (ACR20) at Week 24.
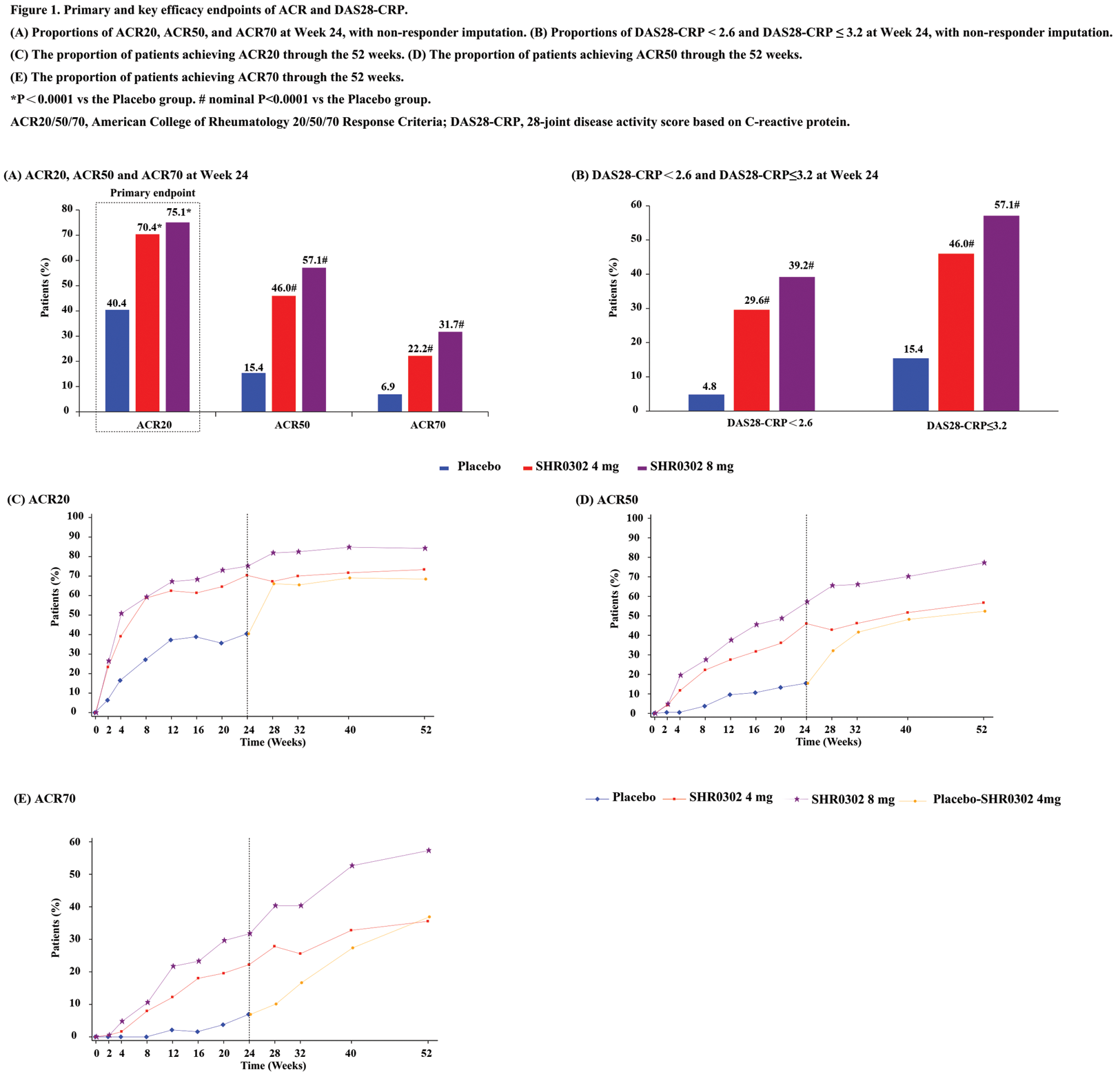
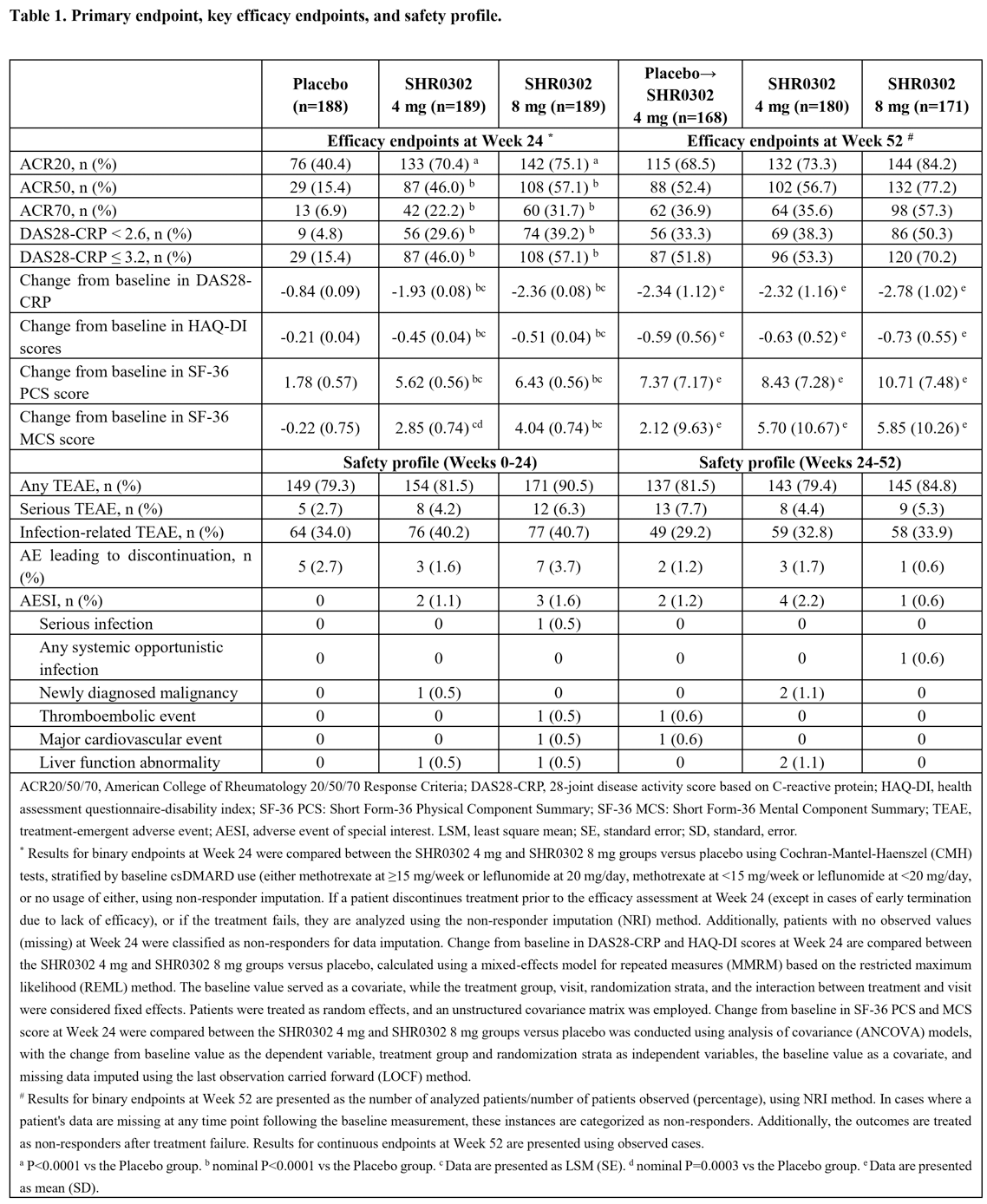
Results: A total of 566 pts were randomized, and all received at least one dose of the study drug. At baseline, demographics and disease characteristics were balanced across treatment arms. Of these, 524 pts (92.6%) completed the 24-week treatment, and 496 pts (87.6%) completed the 52-week treatment. At week 24, the ACR20 response rates were significantly higher for pts receiving SHR0302 at doses of 4 mg (70.4%, P<0.0001) and 8 mg (75.1%, P<0.0001) compared to those on placebo (40.4%). Significant improvements were also observed with SHR0302 at doses of 4 mg and 8 mg compared to placebo in measures of ACR50 and ACR70 responses (Figure 1A). The onset of action was rapid, with obviously more pts in both SHR0302 arms achieving ACR20 at Week 2 compared to the placebo group. Additionally, a significantly higher proportion of pts achieved a DAS28-CRP of < 2.6 and ≤3.2 in the SHR0302 4 mg (29.6% and 46.0%, nominal P<0.0001) and SHR0302 8 mg (39.2% and 57.1%, nominal P<0.0001) groups compared to the placebo group (4.8% and 15.4%) at Week 24 (Figure 1B). At week 24, the SHR0302 4 mg and 8 mg groups exhibited significantly greater changes from baseline in HAQ-DI scores (least squares mean change: -0.45 for 4 mg, nominal P<0.0001; -0.51 for 8 mg, nominal P<0.0001) compared to the placebo group (-0.21). Changes from baseline in the SF-36 score at Week 24 were numerically greater in pts treated with SHR0302 (least squares mean change: PCS: 5.62 for 4 mg and 6.43 for 8 mg, MCS: 2.85 for 4 mg and 4.04 for 8 mg, all nominal P<0.0001) compared to those receiving placebo (PCS: 1.78; MCS: -0.22). These trends in improvements were sustained over an additional 28 weeks (Figure 1 C-E and Table 1). Throughout the 24-week treatment period, the incidences of treatment-emergent adverse events (TEAEs) were 81.5% in the SHR0302 4 mg group, 90.5% in the SHR0302 8 mg group, and 79.3% in the placebo group. The incidence of infection-related TEAEs was slightly higher in the SHR0302 groups (4 mg, 40.2%; 8 mg, 40.7%) than in the placebo group (34.0%). However, few cases of serious infection and systemic opportunistic infection were reported. During this period, one newly diagnosed malignancy in the SHR0302 4 mg group, one thromboembolic event, and one major cardiovascular event in the SHR0302 8 mg group, and two cases of liver function abnormality (one each in the SHR0302 4 mg and 8 mg groups) were reported. The safety profiles for Weeks 0-24 and Weeks 24-52 are presented in Table 1. During the trial, no deaths, tuberculosis cases, or gastrointestinal perforations were reported, and no new safety issues were identified.
Conclusion: Both doses of SHR0302 (4 mg and 8 mg) demonstrated significant and sustained improvements in clinical signs and symptoms in pts with moderately to severely active RA who had an inadequate response to csDMARDs, and exhibited a generally acceptable safety profile.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Xiaofeng Zeng: None declared, Jinjing Liu: None declared, Ying Jiang: None declared, Shangzhu Zhang: None declared, Shengyun Liu: None declared, Jingbo Su: None declared, Changsong Lin: None declared, Xiaohong He: None declared, Rui Wu: None declared, Lei Yang: None declared, Huaxiang Liu: None declared, Xinwang Duan: None declared, Shengqian Xu: None declared, Hui Luo: None declared, Jing Liu: None declared, Qibing Xie: None declared, Guangchao Dong I am employed at Jiangsu Hengrui Pharmaceuticals Co., Ltd., Yuqi Sun I am employed at Jiangsu Hengrui Pharmaceuticals Co., Ltd.