

Background: Emerging evidence suggests that the intestinal microbiota contributes significantly to autoantibodies production in rheumatoid arthritis (RA). Yet, the detailed mechanisms underlying this process remain obscure. Notably, protein posttranslational modifications (PTMs), specifically acetylation, show remarkable conservation across species, ranging from bacteria to humans. Intriguingly, antibodies against post-translationally modified proteins (AMPA) are detectable before RA onset, with acetylated proteins known to induce a broad antibody response against various PTM proteins.
Objectives: This study explores the hypothesis that acetylated proteins in the gut may serve as early antigens, triggering autoantibody production that eventually cross-reacts with joint tissue antigens, potentially accelerating RA onset and exacerbating its severity.
Methods: We employed a collagen-induced arthritis (CIA) model in DBA1 mice, analyzing arthritis severity, incidence, AMPA production, and fecal/intestinal PTM status. Submaximally induced CIA (subCIA) mice were orally administered acetylated or native proteins (derived from E.coli or ovalbumin), followed by comprehensive phenotypic analysis in lymphoid organs and joints. Serum reactivity against joints and intestinal contents was assessed, alongside in vitro experiments focusing on T-cell differentiation and B-cell immunization. Additionally, we evaluated the direct effects of acetylated proteins on the intestinal epithelial barrier using Caco-2 cells.
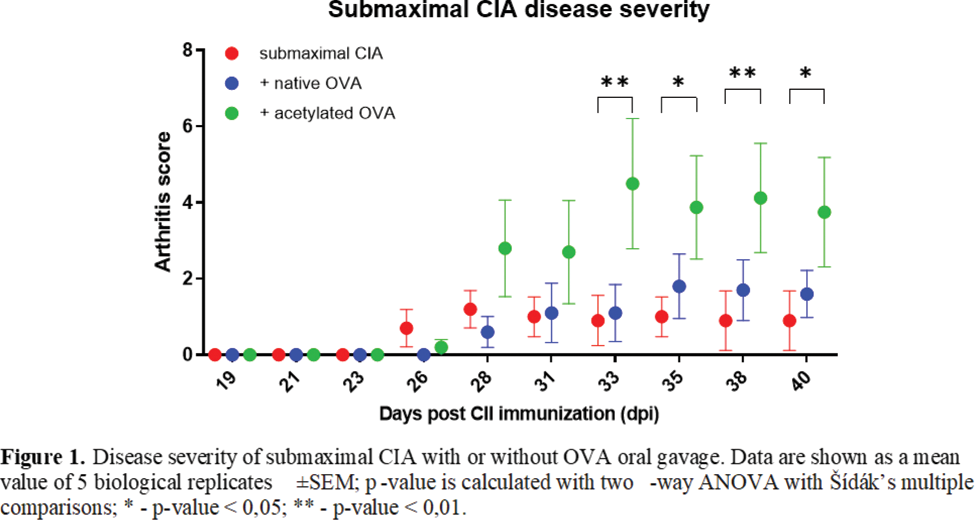
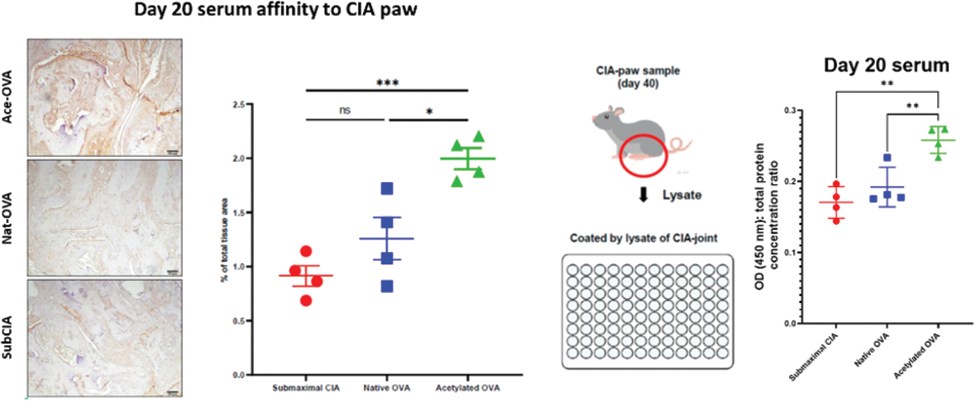
Results: Our findings revealed a time-dependent increase in fecal acetylation, acetylated-lysine area in the colon, and anti-acetyl-vimentin antibodies in CIA mice, notably preceding arthritis onset. Oral administration of acetylated proteins to subCIA mice significantly exacerbated arthritis severity compared to native protein administration ( Figure 1 ). These effects were mirrored in immune response alterations, including increased CD138 + B220 low/- plasma cells and Rorγt + T-cells in the spleen, IL-17A + CD4 + T-cells in the spleen and paw, alongside heightened serum reactivity to the CIA-mouse joints ( Figure 2 ) and intestinal contents. We performed the co-culture of murine bone marrow-derived dendritic cells and isolated T-cells from the spleens - an increase of GATA3 + RORγt + and FoxP3 + -, RORγt + -subsets after acetylated ovalbumin (ace-OVA) stimulation was observed. To verify the induction of AMPA directly, we immunized B-cells in vitro with ace-OVA as described [1]. The supernatants collected from ace-OVA stimulated B-cells showed increased affinity to CIA mouse paw-lysate. For intestinal barrier integrity assessment, we cultured Caco-2 cells with ace-OVA. The concentration of zonulin in the supernatants of ace-OVA-treated cells was significantly higher, which indicated impairment of epithelial integrity. Overall, in vitro experiments corroborated our findings, underscoring the pivotal role of acetylated proteins in driving AMPA production and joint tissue affinity. Finally, using immunoprecipitation with ace-OVA-fed mice serum, we identified one of the target antigens of induced AMPA, which appeared to be vimentin. This finding was confirmed later by mass spectrometry.
Conclusions: This study sheds light on the critical role of acetylated proteins in the intestines as initial antigens for autoantibody production in RA. These early-stage antibodies demonstrate cross-reactivity with joint tissue antigens, suggesting a novel pathway contributing to the disease’s severity. These insights open avenues for innovative therapeutic approaches targeting early autoantibody responses in RA.
REFERENCES: [1] Michelchen S et al. J Immunol Methods 2021.
Acknowledgements: NIL.
Disclosure of Interests: Ippei Miyagawa: None declared, Ilia Gimaev: None declared, Wei Xiang: None declared, Kerstin Dürholz: None declared, Yuichi Maeda: None declared, Eva Schmid: None declared, Heike Danzer: None declared, Britta Eggers: None declared, Sanjukta Gubbi: None declared, Vugar Azizov: None declared, Fabian Schälter: None declared, Michael Frech: None declared, Katrin Marcus-Alic: None declared, Kerstin Sarter: None declared, Yoshiya Tanaka Eli Lilly, AstraZeneca, Abbvie, Gilead, Chugai, Behringer-Ingelheim, GlaxoSmithKline, Eisai, Taisho, Bristol-Myers, Pfizer, Taiho, Mitsubishi-Tanabe, Eisai, Chugai, Taisho, Holger Bang: None declared, Georg Schett: None declared, Mario M. Zaiss: None declared.