

Background: Ianalumab, an afuscosylated monoclonal antibody, depletes B cells by enhanced cellular elimination and blocks survival signals mediated by the B-cell–activating factor receptor (BAFFR). Sjögren’s disease (SjD) is an autoimmune disorder with exocrine glandular and extraglandular manifestations, elevated BAFF and autoantibodies to nuclear antigens. A phase 2b dose-finding trial of ianalumab (NCT02962895) in 190 patients with active Sjögren’s disease met its primary endpoint; 300 mg was clinically efficacious and improved whole salivary flow.
Objectives: To explore changes in serum and saliva protein concentrations in patients with SjD with an aim to characterise biomarkers associated with disease activity and clinical dose response with ianalumab treatment.
Methods: Patients with active SjD (N=190) were randomised (1:1:1:1) to receive placebo or ianalumab (5, 50, or 300 mg). Serum and saliva samples collected at the baseline and week 24 were used for protein profiling and delineation of interferon protein signatures (IFNPS) using the SomaScan(R) v4.1 platform. Levels of autoantibodies, BAFF, B-cell maturation antigen (BCMA), C-X-C motif chemokine ligand 13 (CXCL13) and CCL21 were assessed by immunoassays. A linear mixed-effect model was used to identify longitudinal changes in protein concentration between placebo and treatment groups at week 24 versus baseline. Results were visualised using heatmaps and hierarchical clustering.
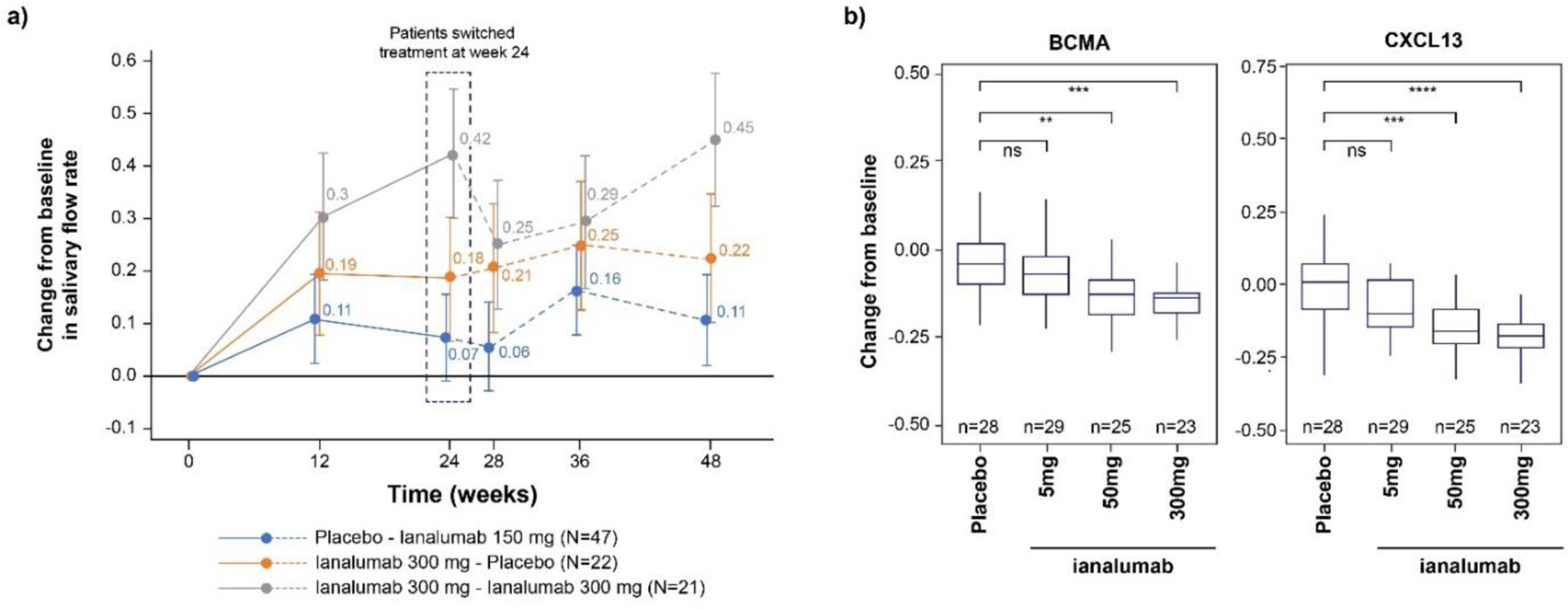
Results: At baseline, cluster analysis did not reveal significant correlations between ESSDAI scores (including subdomains) and levels of autoantibodies or proteins of interest. Ianalumab led to improved salivary flow (Figure 1a) and decreased autoantibody levels, accompanied by change in serum protein levels. The 300-mg dose significantly modulated 42 serum proteins compared to 20 proteins with 50-mg dose and 9 proteins with 5-mg dose after 24 weeks of treatment. This serum protein signature includes B-cell–related markers that were downregulated by ianalumab in a dose-dependent manner. In particular, BCMA expressed by antibody-producing cells, and the chemokines CXCL13 and CCL21, markers of immune infiltration of glandular tissues were further downregulated at 50 mg and 300 mg doses (Figure 1b). Similarly, a trend for downregulation of IFNPS was seen at the higher doses. Finally, some of these changes were also observed in corresponding salivary protein levels.
Conclusion: No differential proteomic signatures related to baseline disease activity were identified. The dose response in clinical efficacy of ianalumab in patients with SjD observed in the phase 2b trial is also reflected at the protein level, with dose-dependent increase in depth and breadth of proteomic changes in serum by week 24. Confirmatory phase 3 trials are currently ongoing (NCT05349214 and NCT05350072).
Dose-dependent modulation of salivary flow rates up to week 48 (a) and validation of two SomaScan signature proteins (BCMA and CXCL13) by immunoassay at week 24 (b )
****0 – 0.0001; ***0.0001 – 0.001; **0.001 – 0.01; ns: 0.05 – 1.
BCMA, B-cell maturation antigen; CXCL13, C-X-C motif chemokine ligand 13; ns, non-significant.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Gwenny M. Verstappen Consultant for Argenx and Aurinia, Hendrika Bootsma Speaker for Novartis and Bristol-Myers Squibb, Consultant for Novartis, Bristol-Myers Squibb, Argenx, Roche, Union Chimique Belge, Unrestricted grant/research support from: Bristol-Myers Squibb, AstraZeneca, Novartis, Stephanie Finzel sponsored talks/courses: Abbvie, Chugai, Galapagos, Novartis, UCB, Consultant of: AstraZeneca, Novartis. Participated in Data Safety Monitoring Board or advisory board of AstraZeneca, Novartis, Received support for attending meetings and/or travel from Janssen, Andrea Grioni Shareholder of: Novartis, Employee of: Novartis, Benjamin Fisher Consultant of: Novartis, Roche, BMS, Galapagos, Janssen, Servier, UCB and Sanofi, Grant/research support from: Janssen, Celgene, Galapagos, Servier, Athena Papas Consultant of: Novartis, Grant/research support from: Novartis, Viela Bio, Exosome Dx, Celine Rauld Shareholder of: Novartis, Employee of: Novartis, Alexandre Avrameas Shareholder of: Novartis, Employee of: Novartis, Danny Tuckwell Employee of: Novartis, Jonas Zierer Shareholder of: Novartis, Employee of: Novartis, Valeria De Luca Shareholder of: Novartis, Employee of: Novartis, Enrico Ferrero Shareholder of: Novartis, Employee of: Novartis, Claire Bonal Shareholder of: Novartis, Employee of: Novartis, Andre da Costa Shareholder of: Novartis, Employee of: Novartis, Rainer Hillenbrand Shareholder of: Novartis, Employee of: Novartis, Isabelle Isnardi Shareholder of: Novartis, Employee of: Novartis, Wolfgang Hueber Shareholder of: Novartis, Employee of: Novartis.