

Background: Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare and severe autoimmune disease, characterized by a pauci-immune necrotizing vasculitis leading to inflammation and damage of major organs. In AAV, autoantibodies against neutrophil cytoplasmic antigens, including proteinase-3 (PR3) or myeloperoxidase (MPO), and the ANCA producing B cells play a central role in the pathogenesis. Autoreactive T cells and their features are less well-studied, but recent studies suggest their importance in the pathogenesis of AAV[1, 2].
Objectives: In this study we aimed to investigate autoreactive T cells and their cytotoxic responses in response to stimulation with ANCA antigens in AAV.
Methods: We investigated the functional responses and features of autoreactive T cells of patients with anti-MPO + AAV (n=8) and anti-PR3 + AAV (n=7), in comparison to healthy controls (HCs) (n=6). 2*10 5 PBMCs were cultured in different conditions (unstimulated, anti-CD3, NP(influenza)/pp65(cytomegalovirus) proteins (50uM each), 1uM MPO or 1uM PR3 antigens) in combination with anti-CD28 for 24h and anti-CD28+IL-2 for 72h. After culturing, T cells were analyzed for activation markers (CD69, GPR56), Granzyme B (GZMB), Granzyme K (GZMK), interferon (IFN)-y responses and proliferation (Ki-67) using flow cytometry. In addition, TCR repertoires and whole transcriptomes of T cells from AAV and HCs were analyzed with single cell RNA sequencing combined with cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq).
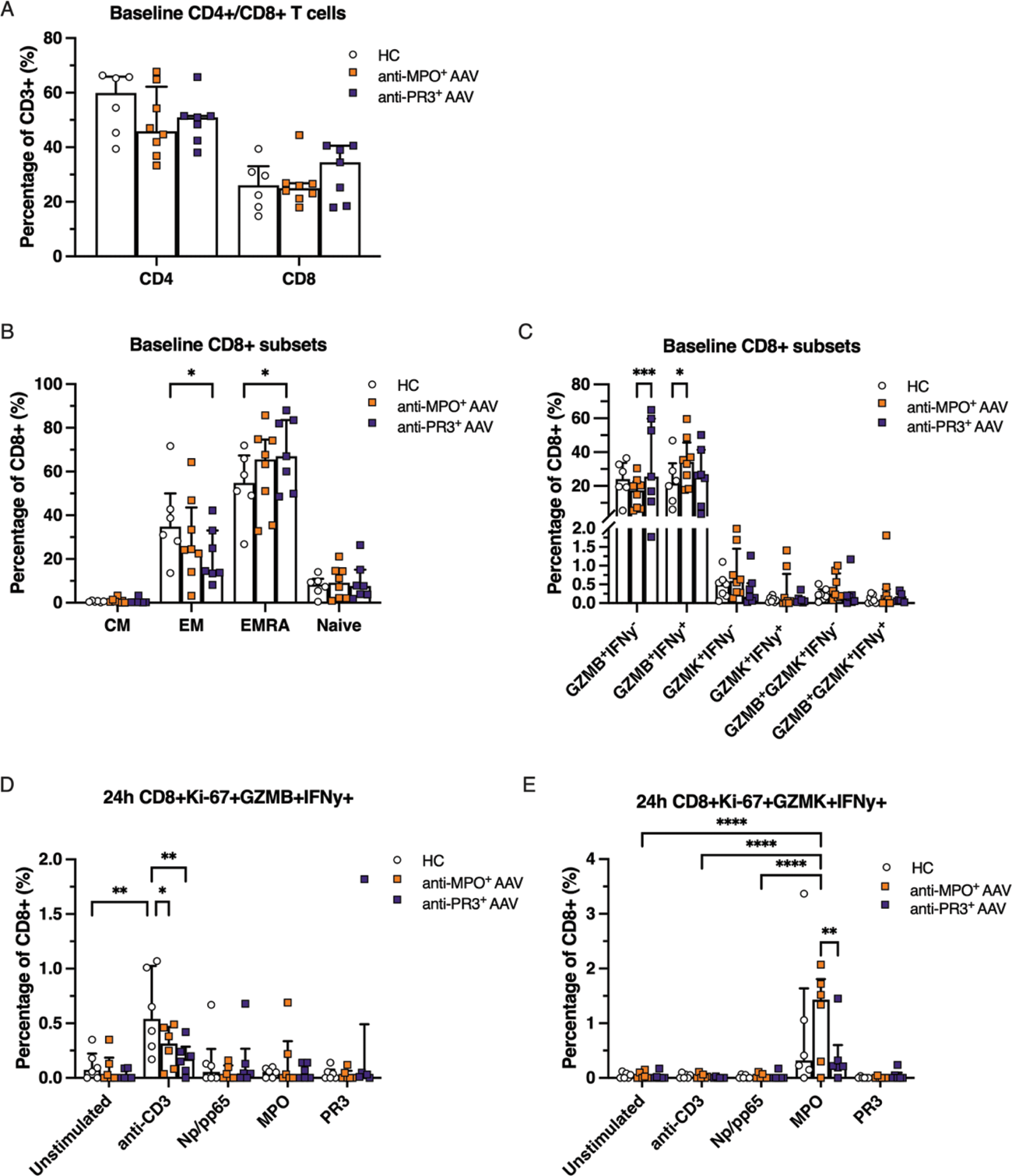
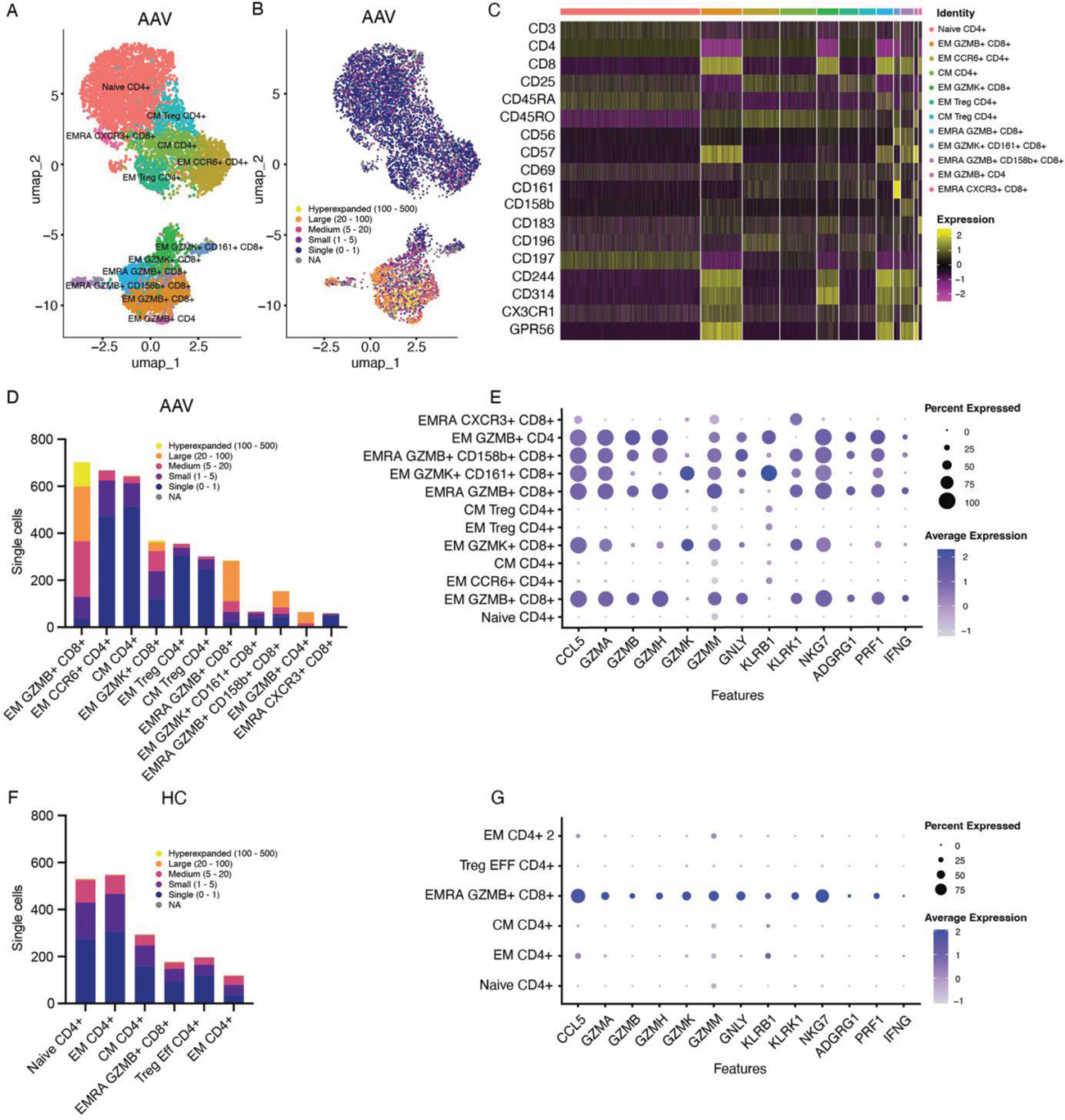
Results: At baseline, the total number of CD4 + and CD8 + T cells did not differ between AAV patients and HCs (Figure 1A). Anti-PR3 + AAV patients exhibited increased effector memory cells re-expressing CD45RA + (EMRA) CD8 + T cells (Figure 1B) and GZMB + CD8 + T cells (Figure 1C), whereas anti-MPO + AAV patients had increased levels of GZMB + IFNy + CD4 + and GZMB + IFNy + CD8 + T cells as compared to HCs (Figure 1C). Stimulation with anti-CD3/anti-CD28, used as a positive control in the culture assay, induced GZMB + IFNy + responses in CD8 + T cells after 24h, which was most pronounced in HCs (Figure 1D). Upon 24h of ANCA antigen stimulation, MPO specifically induced expansion and proliferation of GZMK + IFNy + CD4 + and GZMK + IFNy + CD8 + T cells in anti-MPO + AAV patients, in comparison to anti-PR3 + AAV patients, HCs and other conditions (Figure 1E). Single cell RNA sequencing demonstrated that both anti-MPO + and anti-PR3 + AAV patients had large populations of clonally expanded CD8 + T cells, which were found mostly in the GZMB + but also in the GZMK + CD8 + T cell subpopulations (Figure 2A, B, D), which was not observed in HCs (Figure 2F-G). Representative results of an anti-MPO + AAV patient (A-E) and HC (F-G) is shown in Figure 2. The clonally expanded GZMB + and GZMK + CD8 + T cells in AAV patients were characterized by the surface expression of CD57, CD244 (SLAMF4) and CD314 (NKGD2) (Figure 2C). Specifically, GZMB + CD8 + cells expressed GPR56 and CX3CR1, whereas GZMK + CD8 + T cells were marked by CD56, CD161 (KLRB1) and CD183 (CXCR3) expression (Figure 2C). Moreover, both GZMB + and GZMK + CD8 + T cells of AAV patients displayed effector cytotoxic transcriptional programs, including different granzymes, IFN-y, perforin (PRF1), CCL5, KLRK1 and NKG7 (Figure 2E), which was more abundant than in HCs (Figure 2G).
Conclusion: This study demonstrated that clonally expanded cytotoxic CD8 + T cells are present in both anti-MPO + and anti-PR3 + AAV patients, and we identified a specific autoreactive GZMK + IFNy + CD8 + T cell subpopulation in anti-MPO + AAV patients. The identification and characterization of pathogenic autoreactive T cells in AAV could provide novel insights into next-generation therapeutic targets for this autoimmune disease.
REFERENCES: [1] Mueller, A., et al., Transcriptional and Clonal Characterization of Cytotoxic T Cells in Crescentic Glomerulonephritis. J Am Soc Nephrol, 2023. 34 (6): p. 1003-1018.
[2] Sharma, R.K., et al., Identification of proteinase 3 autoreactive CD4(+)T cells and their T-cell receptor repertoires in antineutrophil cytoplasmic antibody-associated vasculitis. Kidney Int, 2023. 103 (5): p. 973-985.
Acknowledgements: NIL.
Disclosure of Interests: Laura van Dam: None declared, Shady Younis: None declared, Jae-Seung Moon: None declared, Shima Parsafar: None declared, Audra Horomanski Chemocentryx (Honorarium) in 2021, Principia, BeiGene, Gilead (all in 2023), Orr Sharpe: None declared, Jolijn van Leeuwen: None declared, Cees van Kooten: None declared, Y.K. Onno Teng: None declared, William Robinson W.H.R. is a founder and member of the Board of Directors of Atreca Inc., W.H.R. is a consultant of Atreca Inc., Jansen, Sanofi.