

Background: The safety profile of Janus kinase inhibitors (JAKi), particularly concerning infection risks, remains a critical concern in patients with rheumatoid arthritis (RA). Initial pivotal trials and subsequent safety trials[1] showed increased incidences of opportunistic infections and herpes zoster (HZ). However, real-world data on the incidence and severity of these infections continues to be an area of active study.
Objectives: To assess the incidence of infections (any and serious) and HZ in RA patients treated with JAKi, compared to other biologic agents in a large multi-country real-world population.
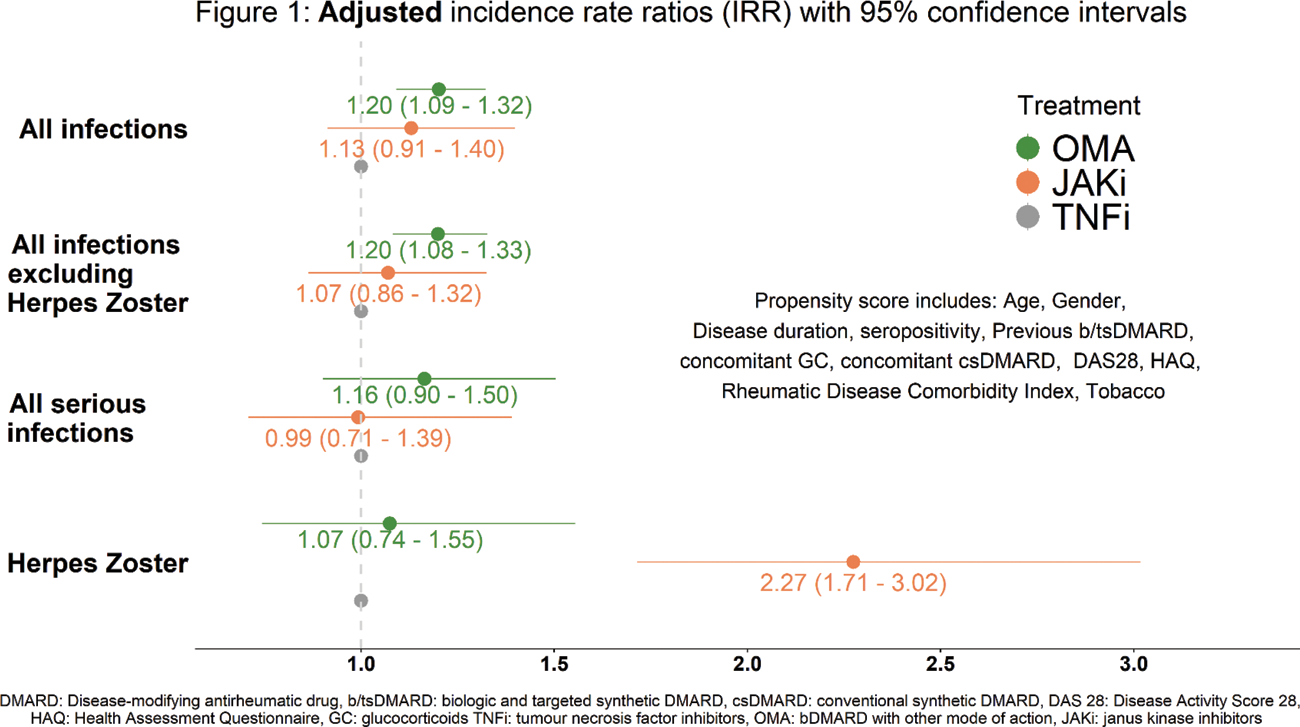
Methods: We studied patients from 14 RA registers across Europe and Québec, starting JAKi, TNF-inhibitors (TNFi) or bDMARDs with other modes of action (OMA). Investigated outcomes included all infections, serious infections (requiring hospitalisation, intravenous treatment or resulting in death), all infections excluding HZ, and HZ. Infections were linked to treatments within 3 months of cessation (1 year after initiation for rituximab) or until follow-up loss, death, or study end. Incidence rates (IR) per 100 patient-years (PY) with 95% confidence intervals (CI) were computed. Poisson regressions with propensity score weighting (including country, disease-, patient-characteristics, and comorbidities, see Figure 1) were performed within each individual register and combined using random-effect meta-analysis to obtain adjusted incidence rate ratios (aIRR) with 95% CI.
Results: Over the 54’905 treatment initiations considered in 36’838 patients with a mean patient follow-up of 2.8 years, 7’070 incident infections were reported of which 1’379 were considered as serious and 352 were HZ. Crude incidence of any infection was lower for TNFi (7.0/100 PY) than for JAKi (9.0/100 PY) and OMA (10.6/100 PY). The adjusted Poisson regression found no significant difference in the incidence of any infections (aIRR = 1.13; 95% CI [0.91; 1.40]) or serious infections (aIRR = 0.99; 95% CI [0.71; 1.39]) between JAKi vs TNFi. However, the incidence of any infection was higher for OMA vs TNFi (aIRR = 1.20; 95% CI [1.09; 1.32]; Figure 1). Compared to TNFi, incidence of HZ was significantly higher for JAKi (aIRR = 2.27; 95% CI [1.71; 3.02]), but not for OMA (aIRR = 1.07; 95% CI [0.74; 1.55]).
Conclusion: In a real-world study with 14 RA registers and all available JAKi, we found no significantly higher risk of infections, either any or serious, in RA patients treated with JAKi compared to TNFi. However, there was a higher risk of any infections with OMA. Compared to TNFi, the incidence of HZ was significantly higher in patients receiving JAKi. Planned subgroup analyses will focus on at-risk populations, specific medications, and infection types to guide treatment choices.
REFERENCES: [1] DOI:10.1056/NEJMoa2109927.
Baseline characteristics
| JAKi
| OMA
| TNFi
|
|
|---|---|---|---|
| Treatment duration, years (median [IQR] ) | 1.4 [0.6; 2.7] | 1.2 [0.4; 2.6] | 1.3 [0.6; 2.8] |
| Age, years (mean (SD )) | 58.3 (12.2) | 60.5 (12.4) | 57.2 (13.3) |
| Female (% ) | 78.3 | 75.8 | 75.5 |
| Disease duration, years (median [IQR] ) | 11.0 [5.4; 18.4] | 11.9 [6.0; 20.0] | 8.4 [3.7; 16.0] |
| Seropositivity (% ) | 80.7 | 84.8 | 77.2 |
| Previous b/ts DMARD (% ) | |||
| 0 | 20.2 | 16.1 | 44.6 |
| 1 | 24.1 | 25.9 | 27.9 |
| 2 | 19.4 | 22.4 | 13.5 |
| ≥ 3 | 36.3 | 35.7 | 13.9 |
| Concomitant csDMARD (% ) | 49.5 | 51.9 | 60.0 |
| Concomitant GC (% ) | 44.2 | 42.0 | 34.4 |
| CRP, mg/L (mean (SD )) | 11.3 (21.2) | 12.2 (27.0) | 11.4 (21.5) |
| CDAI (mean (SD )) | 25.0 (13.7) | 22.2 (14.0) | 23.7 (13.9) |
| DAS 28 (mean (SD )) | 4.7 (1.5) | 4.3 (1.7) | 4.6 (1.6) |
| HAQ (mean (SD )) | 1.2 (0.7) | 1.2 (0.8) | 1.1 (0.7) |
| BMI (mean (SD )) | 27.1 (5.8) | 27.3 (6.0) | 27.3 (6.3) |
| Tobacco (ever) (% ) | 36.0 | 38.7 | 38.5 |
csDMARDs = conventional synthetic DMARDs, GC = glucocorticoids, CRP = C-reactive protein, CDAI = Clinical Disease Activity Index, DAS 28 = Disease Activity Score 28, HAQ = Health Assessment Questionnaire, BMI = Body Mass Index.
Acknowledgements: NIL.
Disclosure of Interests: Romain Aymon: None declared, Denis Mongin: None declared, Benoit Gilbert: None declared, Romain Guemara: None declared, Denis Choquette Abbvie, Amgen, Pfizer, Sandoz and Tevapharm, Abbvie, Amgen, Celltrion, Eli Lilly, Fresenius-Kabi, INESSS, Jamppharma, Pfizer, Sandoz and Tevapharm, Rhumadata is supported through grants from Abbvie, Amgen, Fresenius-Kabi, Eli Lilly, Pfizer, Sandoz and Tevapharm, Catalin Codreanu Abbvie, Amgen, Boehringer Ingelheim, Ewopharma, Lilly, Novartis, Pfizer, AbbVie, Amgen, Boehringer Ingelheim, Ewopharma, Lilly, Novartis, Pfizer, Irini Flouri: None declared, Doreen Huschek: None declared, Kimme Hyrich: None declared, Florenzo Iannone Abbvie, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, SOBI, Roche and UCB, Abbvie, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, SOBI, Roche and UCB, Lianne Kearsley-Fleet: None declared, Tore K. Kvien AbbVie, Amgen, Celltrion, Gilead, Grünenthal, Novartis, Pfizer, Sandoz, UCB, AbbVie, Amgen, Celltrion, Gilead, Grünenthal, Novartis, Pfizer, Sandoz, UCB, Burkhard F. Leeb Eli-Lilly, Pfizer, Astropharma, Biogen, Celgene, Eli-Lilly, Pfizer, AbbVie, Biogen, Celgene, Dan Nordström Abbvie, BMS, Lilly, MSD, Novartis, Pfizer, Roche, UCB, Karel Pavelka: None declared, Manuel Pombo-Suarez Janssen, Merck Sharp & Dohme, Novartis and Sanofi Genzyme, Sella Aarrestad Provan: None declared, Ana Maria Rodrigues Amgen, Pfizer, Astrazeneca, Novartis, Roche and Nordic pharma, Amgen, Pfizer, Astrazeneca, Novartis, Roche and Nordic pharma, Reuma.pt is supported througth grants from Abbvie, Bristol Myers Squibb, Lilly, MSD, Novartis, Pfizer, Boehringer Ingelheim, Astrazeneca and Amgen, Ziga Rotar Abbive, Amgen, Biogen, Eli Lilly, Medis, Mediasi, Novartis, Sandoz, Pfizer, MSD, Sanofi, Roche, SOBI, Abbive, Amgen, Biogen, Eli Lilly, Medis, Mediasi, Novartis, Sandoz, Pfizer, MSD, Sanofi, Roche, SOBI, Prodromos Sidiropoulos AbbVie, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Amgen and UCB, AbbVie, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Amgen and UCB, AbbVie, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Amgen and UCB, Anja Strangfeld Abbvie, Amgen, BMS, Celltrion, MSD, Lilly, Pfizer, Roche, UCB, AbbVie, Amgen, BMS, Celltrion, Fresenius Kabi, Galapagos, Hexal, Lilly, MSD, Pfizer, Samsung Bioepis, Sanofi Aventis, Viatris Sante, and UCB, and previously Roche. The German Biologics Register RABBIT is supported by a joint unconditional grant from these companies., Nina Trokovic: None declared, Jakub Zavada Abbvie, Elli-Lilly, Sandoz, Novartis, Egis, UCB, Sanofi, Astra Zeneca, Sobi, Abbvie, Elli-Lilly, Sandoz, Novartis, Egis, UCB, Sanofi, Astra Zeneca, Sobi, Delphine Sophie Courvoisier: None declared, Axel Finckh AbbVie, Eli-Lilly, Novartis, MSD, Pfizer, UCB, BMS, Eli-Lilly, Pfizer, AbbVie, BMS, Eli-Lilly, Galapagos, Pfizer, Kim Lauper Pfizer, AbbVie, Eli-Lilly, Galapagos and Pfizer.