

Background: Osteitis and bone microstructure changes (BMC) are known to be the earliest radiographical signs of rheumatoid arthritis (RA) that precede the development of bone erosion. Recently, a JAK inhibitor, Baricitinib (BAR), was shown to inhibit these early changes. However, the underlying molecular mechanisms are not fully identified.
Objectives: This study aimed to reveal the underlying molecular mechanism of osteitis and BMC, including the role of JAK-STAT cytokines using SKG mice, an animal model of RA with a ZAP70 gene point mutation.
Methods: Arthritis was induced by intraperitoneal injection of Zymosan. BAR or a vehicle was orally administered in Zymosan-SKG mice. Osteitis was assessed by an increase of the femur/gastrocnemius muscle (F/G) ratio in fat-suppressed T2-weighted images on MRI. BMC was evaluated by a decrease of bone volume/tissue volume (BV/TV) ratio on micro CT. Immunohistochemistry (IHC) was performed to identify morphological changes and the distribution of tartrate-resistant acid phosphatase (TRAP)-positive cells in the BM. Flow cytometry (FCM) was used to analyze the bone marrow (BM) cells, including granulocyte-monocyte (GM) progenitor cells, granulocytes, and monocytes. The role of JAK-STAT cytokines was investigated by examining the expression of these cytokines in the BM of arthritis mice. Additionally, recombinant (r-) GM-CSF was supplemented in both in vivo arthritis model and in vitro RANKL-mediated osteoclast differentiation assay using mice and sorted human CD14+ monocyte. Expression of GM-CSF related genes in the BM from RA and osteoarthritis (OA) patients were compared using public database (Lee, Arthritis Res Ther 2011).
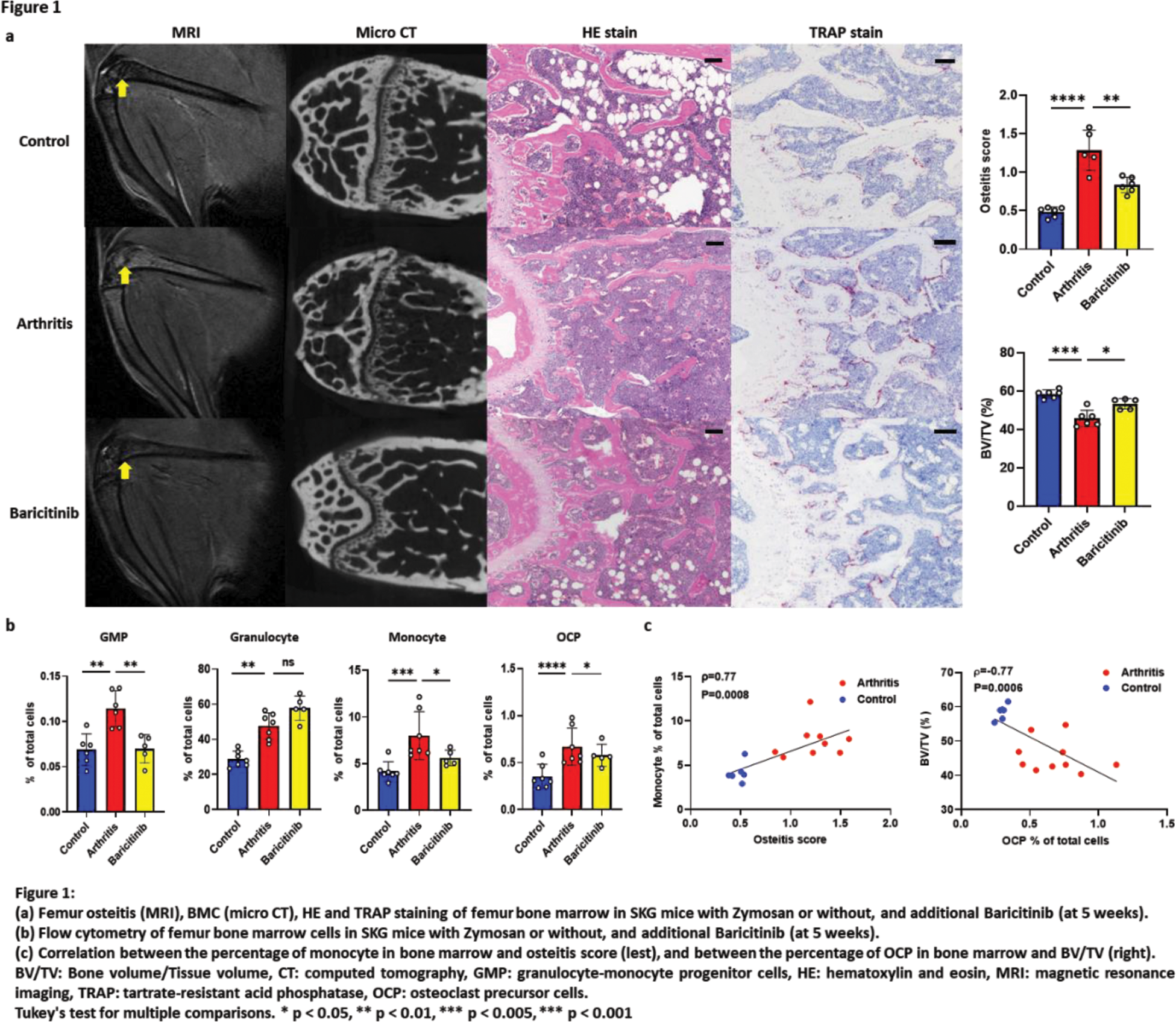
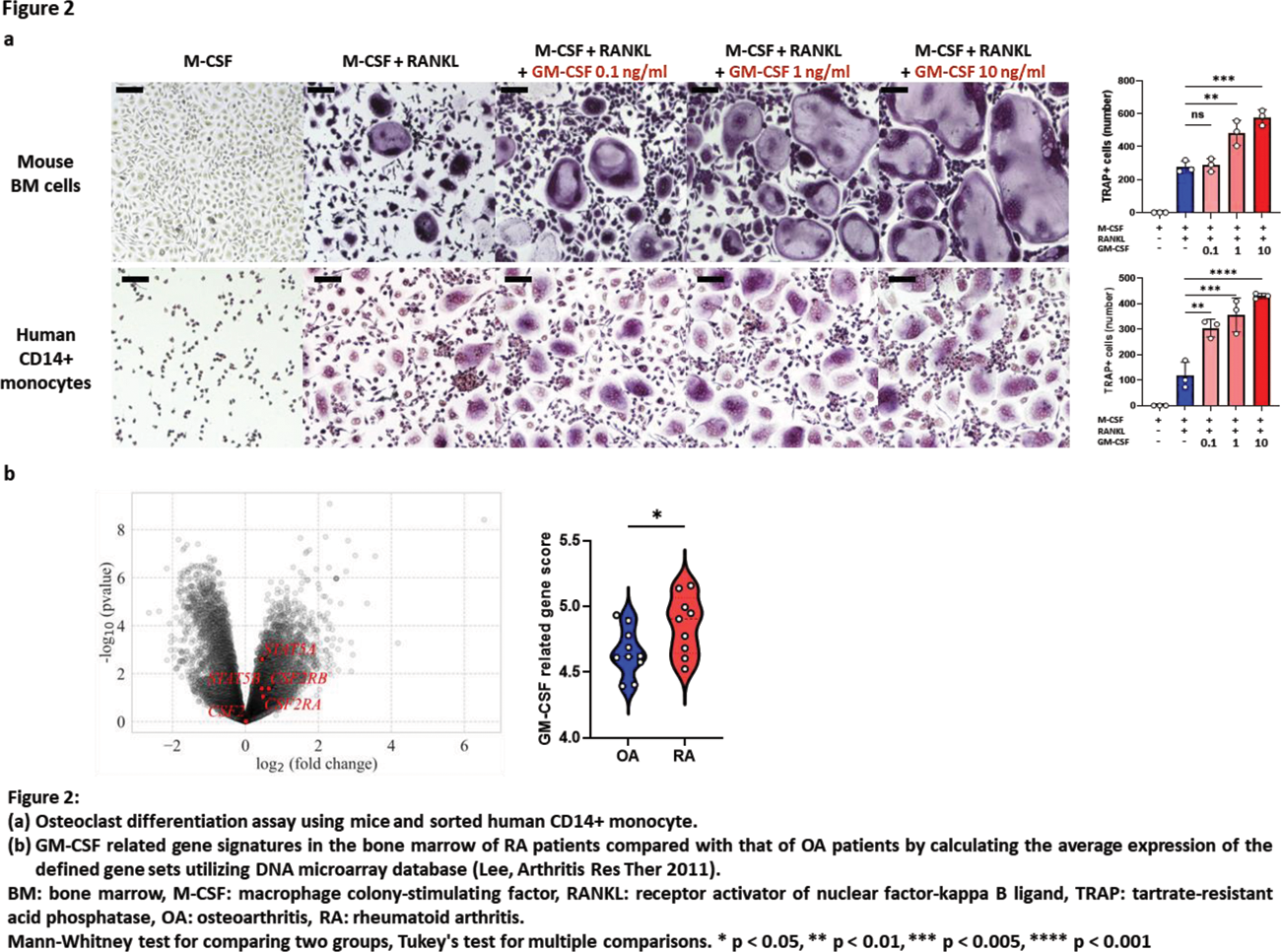
Results: Following zymosan injection, both osteitis and BMC were significantly exacerbated in the arthritis model (Figure 1a). Notably, osteitis appeared earlier, at 2 weeks, during the progression of arthritis, followed by the subsequent appearance of BMC in the same region. Osteitis scores on MRI also well-correlated with BMC scores on micro CT (r=-0.58, p=0.03). IHC revealed an increase in BM cells, including TRAP-positive cells which were located where osteitis and BMC were observed (Figure 1a). FCM showed an increase in GM progenitor cells, granulocytes, monocytes, and osteoclast precursor cells in the BM (Figure 1b). The number of monocytes significantly correlated with osteitis scores (r=0.77, p=0.0008), and the number of osteoclast precursor cells correlated with worsening of BMC (r=-0.76, p=0.0006) (Figure 1c). BAR effectively suppressed osteitis (vs arthritis p=0.003) and BMC (vs arthritis p=0.03) (Figure 1a). BAR also decreased the number of GM progenitor cells, monocytes, and osteoclast precursor cells (Figure 1b). Among JAK-STAT cytokines, GM-CSF was highly expressed in the BM of arthritis mice. In vivo administration of rGM-CSF to Zymosan-SKG exacerbated osteitis and BMC. In vitro addition of rGM-CSF to mouse BM cells markedly increased the number and size of osteoclasts, especially when added in the late phase of osteoclast differentiation. The same phenomenon was observed upon adding rGM-CSF to CD14+ human monocyte cultures (Figure 2a). BAR inhibited the osteoclast differentiation-accelerating effect of rGM-CSF. Expression of GM-CSF related genes (including STAT5a , STAT5b ) in the BM were significantly higher in RA patients compared to OA patients (p=0.04) in public database (Figure 2b).
Conclusion: This study elucidated that JAK-STAT cytokines, especially GM-CSF, play a crucial role in the development of osteitis and BMC by increasing of GM-lineage cells and osteoclast precursor cells. Baricitinib may suppress the progression of osteitis and BMC through inhibition of GM-CSF signaling.
REFERENCES: NIL.
Acknowledgements: We would like to sincerely thank all those who have been involved in this study for their valuable contributions.
Disclosure of Interests: Tsuneyasu Yoshida Chugai Pharmaceutical Co., Asahi Kasei Pharma Co., UCB Japan Co., Yoshiki Murotani: None declared, Koichi Murata: None declared, Hirohiko Imai: None declared, Takeshi Iwasaki: None declared, Yoichi Nakayama: None declared, Masao Katsushima: None declared, Mirei Shirakashi: None declared, Ran Nakashima: None declared, Ryu Watanabe Asahi Kasei, Chugai, Eli Lilly, GSK, and UCB Japan., AbbVie., Kosaku Murakami: None declared, Hajime Yoshifuji: None declared, Akio Morinobu AbbVie Co., Bristol-Myers Squibb Co., Chugai Pharmaceutical Co., Mitsubishi Tanabe Pharma Co., Eli Lilly Japan Co., Astellas Pharma Co., Eisai Co., Gilead Sciences Japan Co., Pfizer Co., AbbVie Co., Mitsubishi Tanabe Pharma Co., Asahi Kasei Pharma Co., Eisai Co., Chugai Pharmaceutical Co., Motomu Hashimoto Brystol Meyers, Chugai, Eisai, Eli Lilly, and Tanabe Mitsubishi., AbbVie, Asahi Kasei, Astellas, Brystol Meyers, Eisai, Daiichi Sankyo, Eli Lilly, Novartis Pharma, and Taisho Toyama.