

Background: In the complex panorama of autoimmune diseases, characterization of pivotal pathogenic autoantibodies involved in disease progression remains challenging.
Objectives: This study aimed to identify novel antibodies through a global antibody profiling strategy and elucidate the pathological implications and clinical significance of identified autoantibodies in systemic sclerosis (SSc).
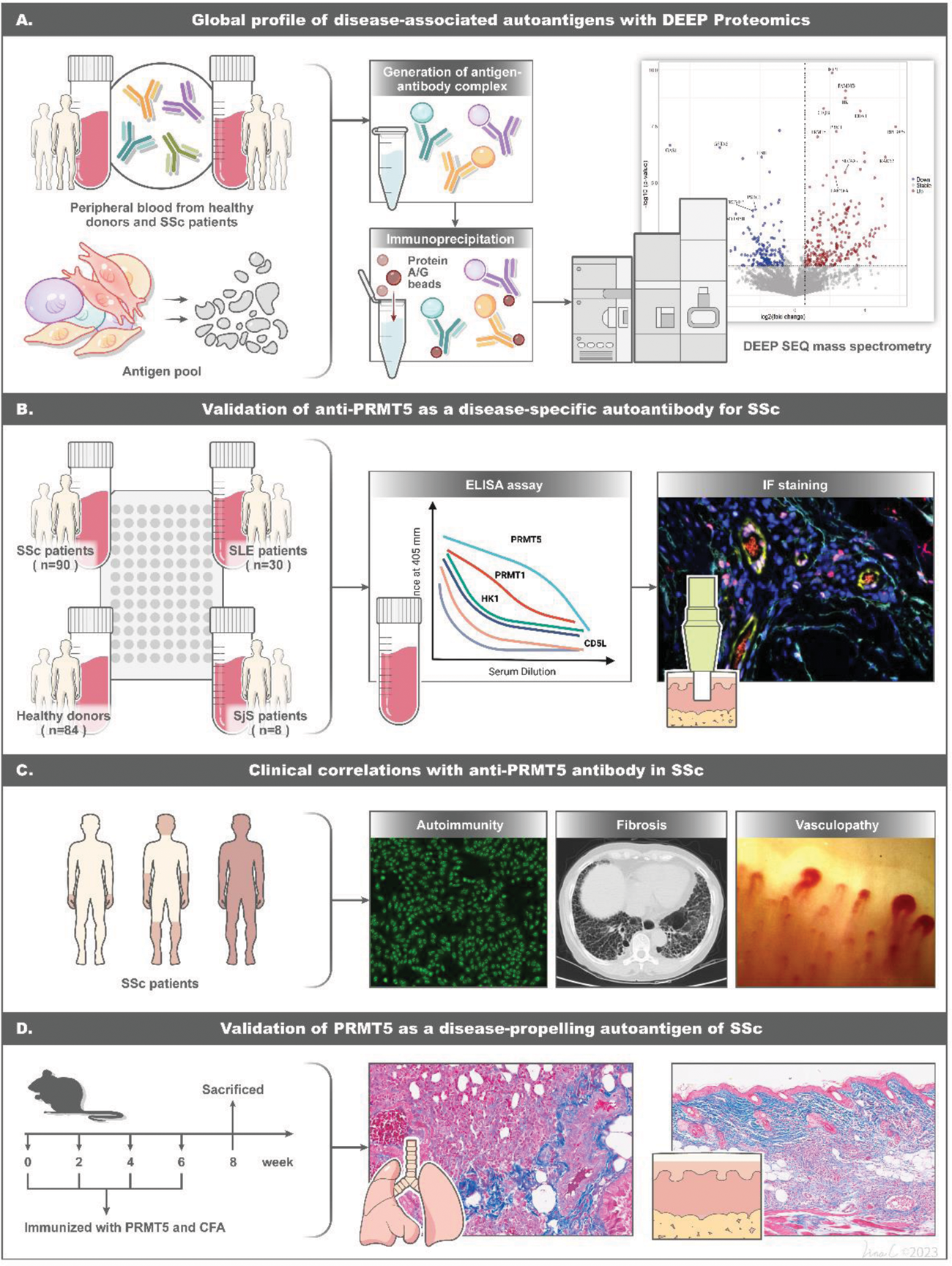
Methods: We implemented this strategy by conducting immunoprecipitation on the whole IgG/IgA binding proteins from SSc and healthy sera, utilizing antigen pools derived from extracts of cells, including human umbilical vein endothelial cells (HUVECs), human dermal fibroblasts (HDFs), Jurkat T cells and THP-1 monocytes. IgG/IgA binding proteins were proceeded with quantitative proteomics and over-presented by bioinformatics analysis. The identified antibodies were then orthogonally validated in a SSc cohort. Mice were immunized with the target antigen, and subsequent evaluation was conducted through histological examination and RNA-sequencing.
Results: We detected 238 significantly upregulated IgG/IgA binding proteins. Amongst those, the concentrations of anti-PRMT5 antibodies were significantly elevated in SSc patients, demonstrating robust diagnostic accuracy in distinguishing SSc patients from healthy controls and other autoimmune diseases including Systemic Lupus Erythematosus (SLE) and Sjögren’s Syndrome (SjS) with an area under the curve (AUC) of 0.900 to 0.988. Notably, 31.11% of SSc patients tested positive for anti-PRMT5 antibodies, and the correlation of their levels with the trajectory of disease progression or regression was assessed in the context of both skin and lung fibrosis. The pathogenetic effects of these antibodies were proven by immunization experiments. Mice immunized with PRMT5 displayed significant inflammation and fibrosis in both skin and lungs, accompanied by the enrichment of multiple proinflammatory and profibrotic pathways on RNA-seq.
Conclusion: This study has identified anti-PRMT5 antibodies as a novel autoantibody in SSc and demonstrated their role as drives of inflammation and fibrotic tissue remodeling.
Overview of experimental workflow and key discoveries
REFERENCES: [1] Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009;360(19):1989-2003. doi: 10.1056/NEJMra0806188.
[2] Denton CP, Khanna D. Systemic sclerosis. Lancet 2017;390(10103):1685-99. doi: 10.1016/s0140-6736(17)30933-9 [published Online First: 20170413].
Acknowledgements: NIL.
Disclosure of Interests: None declared.