

Background: Systemic sclerosis (SSc) is a complex immune-mediated disease characterized by immune dysregulation, vascular damage and fibrosis. Its onset is influenced by many factors, including genetics. Notably, sex plays a pivotal role with higher prevalence in females than males (8:1 ratio) and different clinical manifestations between sexes. However, the underlying mechanisms of such sex differences have been unexplored and remain poorly understood, contributing to health care disparities for women worldwide. Genomic studies have revealed pathways associated with disease onset and progression, thus including the analyses of these sex-differences will represent a significant step towards precision medicine in SSc, ensuring equitable medicine for all, especially in a disease with such a marked sex bias.
Objectives: A comprehensive analysis of the influence of sex in SSc may reveal novel pathogenic mechanisms with sex-specific or sex-differential effects. The objective of this study was to assess genetic risk factors contributing to the sexual dimorphism in SSc through a sex-interaction genome-wide association study (GWAS).
Methods: This assessment leveraged the largest genetic study in SSc described to date, involving 9,095 patients (7,811 females and 1,282 males) and 17,584 controls (10,691 females and 6,893 males). After genotype imputation and quality controls, the differential effect between sexes of 9 million genetic variants was analyzed through a Genotype by Sex interaction term. Gene prioritization of significant (p<5E-8) and suggestive (p<5E-6) associated loci was carried out employing OpenTargets database.
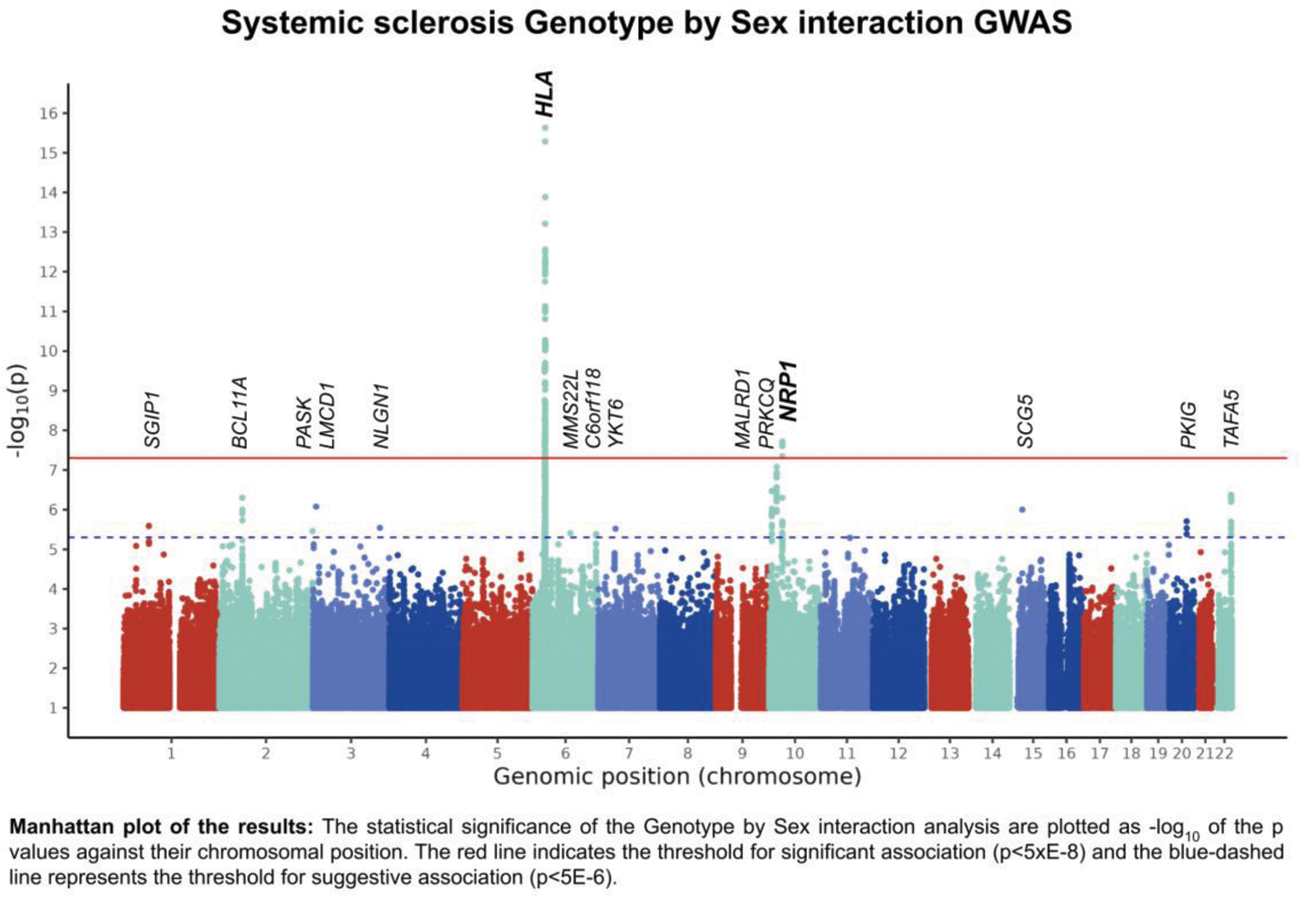
Results: We identified a novel non-HLA genome-wide significant association with SSc risk with differential effect between females and males (Figure 1). This association mapped in the vicinity of Neuropilin 1 ( NRP1 )(rs2812627, p Interaction = 2.49E-8, OR Interaction = 0.57). We further analyzed the effect of this locus for each sex through a stratified analysis, revealing a suggestive risk effect for males and a nominal association for females in the opposite direction (p Females = 0.009, OR Females = 0.88; p Males = 2.90E-6, OR Males = 1.52). By leveraging public databases, we observed that the significantly associated genetic variants overlap with transcriptional regulatory mechanisms in several immune cell types. In this regard, we found an alteration of expression through the eQTL mechanism of ITGB1 in Natural Killer cells and of NRP1 in blood tissue. NRP1 is involved in endothelial cell function, interacting with the vascular endothelial growth factor. Indeed, altered levels of NRP1 have been reported in vascular involvement in SSc[1]. Additionally, NRP1 is a marker for a subset of T regulatory cells and has been linked to their function, which is crucial for immune homeostasis. Other studies have also linked NRP1 with non-regulatory T cells recognizing self-antigens[2,3]. Given the differences in T cell number and profile between sexes[4], it is plausible that these distinctions contribute to the differential impact of NRP1 in the disease between males and females.
Conclusion: Using the largest SSc cohort and a genome-wide approach, we have found evidence for a genetic component involved in SSc sex dimorphism. Moreover, this genetic component involves a novel risk locus for the disease.
REFERENCES: [1] Romano, E. et al. Decreased expression of neuropilin-1 as a novel key factor contributing to peripheral microvasculopathy and defective angiogenesis in systemic sclerosis . Annals of the Rheumatic Diseases 75, 1541–1549 (2015).
[2] Raveney, B. J. et al. Neuropilin-1 (NRP1) expression distinguishes self-reactive helper T cells in systemic autoimmune disease. EMBO Molecular Medicine 14, (2022).
[3] Kalekar, L. A. et al. CD4+ T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nature Immunology 17, 304–314 (2016).
[4] Klein, S. L. & Flanagan, K. L. Sex differences in immune responses . Nature Reviews Immunology 16, 626–638 (2016).
Acknowledgements: We would like to thank Sofia Vargas and Gema Robledo for their excellent technical assistance. We also appreciate the essential collaboration of all the patients and healthy donors who provided the data for these studies. This research was funded by Redes de Investigación Cooperativa Orientadas a Resultados en Salud (RICORS) (RD21/0002/0039) from Instituto de Salud Carlos III (ISCIII). I.R.M and C.R.B were supported by the program FPU: (FPU21/02746) and (FPU22/01652) respectively, funded by the Spanish Ministry of University. L.O.F. work was supported by a Juan de la Cierva Incorporacion fellowship (IJC2019-040746-I) funded by MCIN/AEI/10.13039/501100011033. M.A.H. is a recipient of a Miguel Servet fellowship (CP21/00132) from ISCIII. This research is part of the doctoral degree awarded to I.R.M., within the Biomedicine program from the University of Granada.
Disclosure of Interests: None declared.