

Background: Although microvascular changes are the earliest histopathologic manifestation of systemic sclerosis (SSc), the vascular pathophysiology and the microenvironment within vascular niche remains poorly understood.
Objectives: Here, we employed three different spatial omics approaches to phenotype changes of endothelial cell populations and of the vascular niche in the skin of SSc patients and healthy individuals.
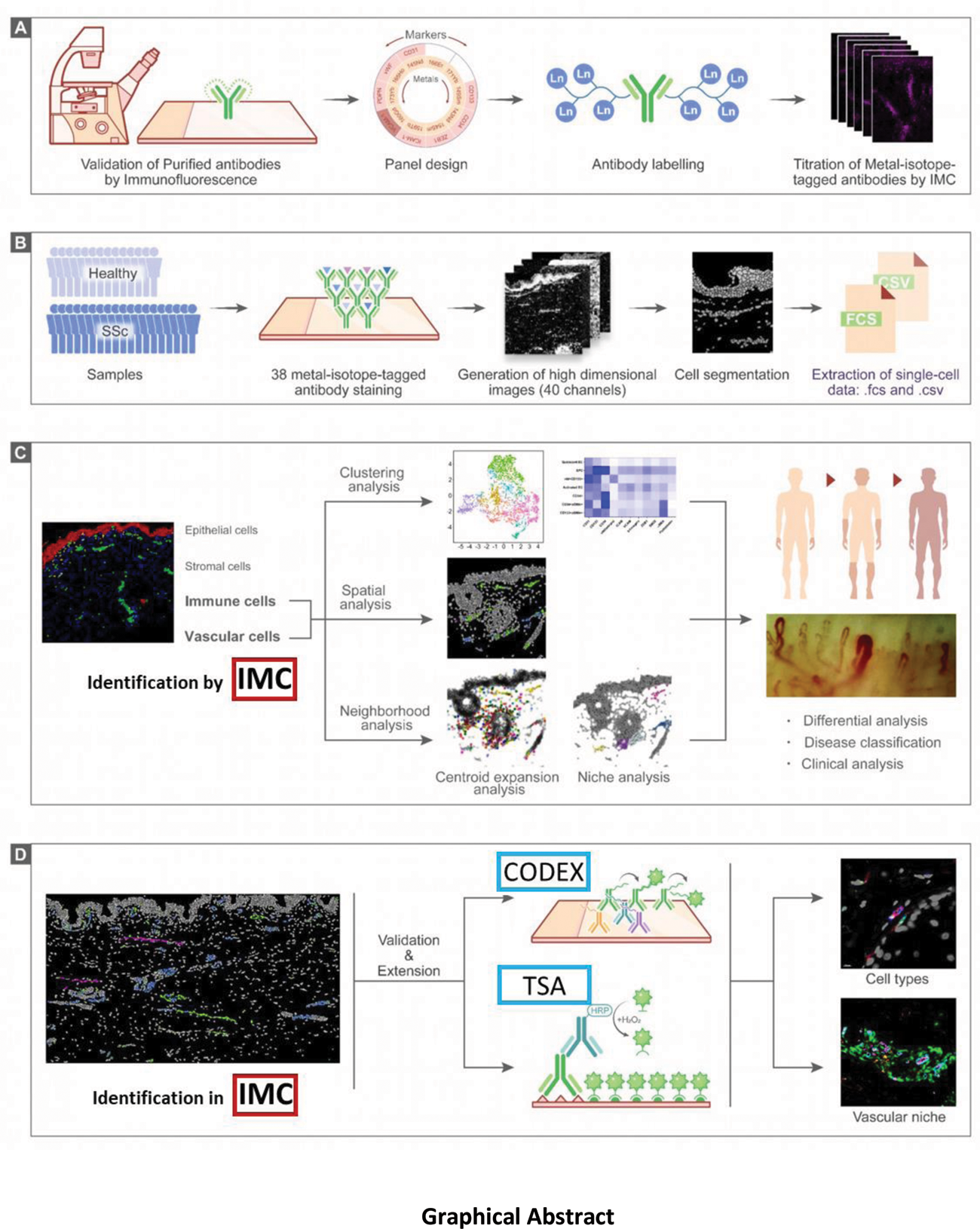
Methods: Imaging mass cytometry (IMC) was applied to deconvolute the heterogeneity of vascular cells at single-cell level in situ and to characterize cellular alterations of the vascular niches of SSc patients. Our IMC antibody panel was designed to target epitopes specific for different subsets of endothelial cells, endothelial precursor cells, immune cells and mesenchymal cells, as well as structural markers used for tissue segmentation. We performed clustering analysis and neighborhood analysis based on single-cell data extracted from IMC. To further corroborate the identified cell types by IMC with techniques with higher resolution, we additionally performed Co-Detection by Indexing (CODEX) and Tyramide Signal Amplification (TSA).
Results: Skin biopsies of SSc patients and healthy individuals were analyzed, yielding a total of 90,755 cells including 2,987 endothelial cells and 4,096 immune cells. We identified seven different subpopulations of blood vascular endothelial cells (VECs), two subpopulations of lymphatic endothelial cells (LECs) and three subpopulations of pericytes. A novel population of CD34 + ;αSMA + ;CD31 + VECs was more common in SSc, whereas endothelial precursor cells (EPCs) were decreased in SSc. The microenvironment of CD34 + ;αSMA + ;CD31 + VECs was enriched for immune cells and myofibroblasts, and CD34 + ;αSMA + ;CD31 + VECs expressed markers of endothelial-to-mesenchymal transition (EndMT). Higher density of CD34 + ;αSMA + ;CD31 + VECs in SSc skin was associated with an increase in modified Rodnan skin score (mRSS) ≥ 25% from baseline on the follow-up visits (12 ± 2 months after baseline) and a higher EUSTAR activity index. Moreover, the number of monocytes in the vascular niche correlated with the progression of skin fibrosis measured as increase in mRSS and the EUSTAR activity index.
Conclusion: Using multiple spatial proteomic approaches, we unraveled the heterogeneity of vascular cells in healthy individuals and SSc patients. We identified CD34 + ;αSMA + ;CD31 + VECs as a novel EC population that is increased in SSc patients, expresses EndMT markers and is located in close proximity to immune cells and myofibroblasts. CD34 + ;αSMA + ;CD31 + VEC counts and monocyte counts in vascular niche were associated with clinical outcomes of progressive fibrotic remodeling, thus providing a novel cellular correlate for the crosstalk of vasculopathy and fibrosis.
REFERENCES: [1] Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685-1699. doi: 10.1016/S0140-6736(17)30933-9.
[2] Distler JHW, Gyorfi AH, Ramanujam M, Whitfield ML, Konigshoff M, Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. 2019;15:705-730. doi: 10.1038/s41584-019-0322-7.
[3] Giesen C, Wang HA, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, Schuffler PJ, Grolimund D, Buhmann JM, Brandt S, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. 2014;11:417-422. doi: 10.1038/nmeth.2869.
[4] Jackson HW, Fischer JR, Zanotelli VRT, Ali HR, Mechera R, Soysal SD, Moch H, Muenst S, Varga Z, Weber WP, Bodenmiller B. The single-cell pathology landscape of breast cancer. Nature. 2020;578:615-620. doi: 10.1038/s41586-019-1876-x.
[5] Moldoveanu D, Ramsay L, Lajoie M, Anderson-Trocme L, Lingrand M, Berry D, Perus LJM, Wei Y, Moraes C, Alkallas R, et al. Spatially mapping the immune landscape of melanoma using imaging mass cytometry. Sci Immunol. 2022;7:eabi5072. doi: 10.1126/sciimmunol.abi507.
Graphical Abstract
Acknowledgements: We thank Regina Kleinlein, Christoph Liebel, Dorothee Köhler, and Vladyslav Fedorchenko for excellent technical assistance. We also wish to acknowledge the expertise of Christina Schniederjohann in CODEX.
Disclosure of Interests: Jörg Distler JHWD is stock owner of 4D Science and Scientific Leader of FibroCure., Although no disclosures directly relate to the present study, JHWD has consultancy relationships with AbbVie, Active Biotech, Anamar, ARXX, AstraZeneca, Bayer Pharma, Boehringer Ingelheim, Celgene, Galapagos, Genentech, GSK, Inventiva, Janssen, Novartis, Pfizer, Roche and UCB., JHWD has received research funding from Anamar, Argenx, ARXX, BMS, Bayer Pharma, Boehringer Ingelheim, Cantargia, Celgene, CSL Behring, Galapagos, GSK, Inventiva, Kiniksa, Lassen, Sanofi-Aventis, RedX, UCB., Minrui Liang: None declared.