

Background: Cardiovascular diseases account for about 15% of the causes of death in patients with rheumatoid arthritis (RA) [1], and prediction and prevention of major adverse cardiovascular events (MACE) in patients with RA is important. Existing cardiovascular event prediction scores developed for the general population underestimate risk when applied to patients with RA [2]. Therefore, the development of a more accurate prediction algorithm is urgently needed. Despite its widespread use in the assessment of heart disease, the cardiothoracic ratio (CTR) has not been used in any cardiovascular event prediction scores to date. Incorporating radiograph indices like CTR into existing scores may alter the predictive score for patients with RA.
Objectives: To investigate whether incorporating radiograph indices into existing cardiovascular risk prediction scores enhances the predictive accuracy for MACE in patients with RA.
Methods: Patients aged ≥20 with RA, enrolled in the Institute of Rheumatology RA (IORRA) cohort, and with chest and hand radiograph data from April 2000 to September 2022 were included. MACE, defined as the occurrence of myocardial infarction (MI), heart failure, stroke, or cardiovascular-related hospitalization or death, was captured by self-reported items by patients in the IORRA database. Observations began at the earliest radiographic date and censored at the occurrence of MACE, loss to follow-up, 10 years after observation initiation, or September 2023, whichever came first. The CTR and tracheal bifurcation angle (TBA) were extracted using a heatmap regression model trained on a dataset of 1,400 National Institute of Health chest radiograph images. The Sharp/van der Heijde score (SHS) of hand radiographs was extracted using the method reported in our previous study [3]. Using a time-dependent Cox regression model with relevant covariates, the associations of these radiograph indices (i.e., CTR, TBA, and SHS) with MACE were assessed. The event-free survival rate for MACE according to CTR tertile was described by the Kaplan-Meier method and compared by log-rank test. To incorporate a score for CTR tertile into existing scores, namely the modified Systematic Coronary Risk Evaluation (mSCORE) or Expanded Cardiovascular Risk Prediction Score for RA (ERS-RA), patients were randomly assigned in a 1:1 ratio to either the Derivation or Validation cohort, with the number of points to be added to the score determined by using the coefficient from the Penalized Cox regression model in the Derivation cohort. Subsequently, the predictive accuracy in the Validation cohort was assessed using area under the curve (AUC).
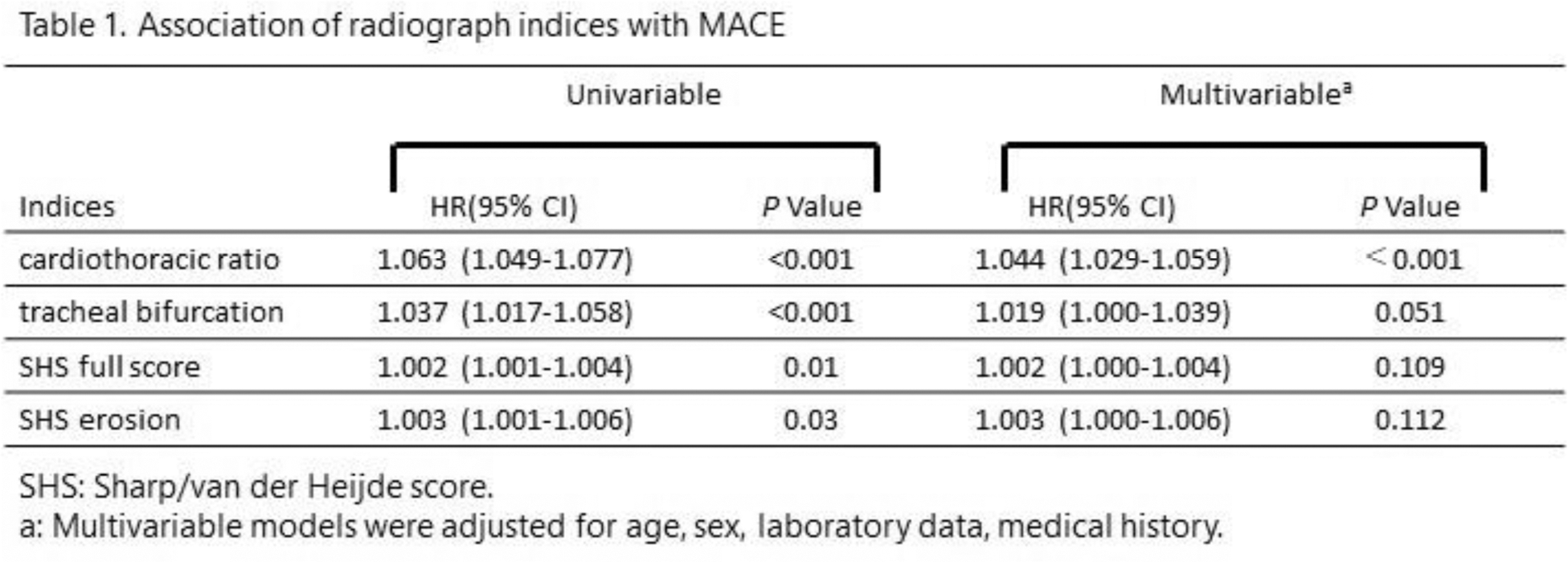
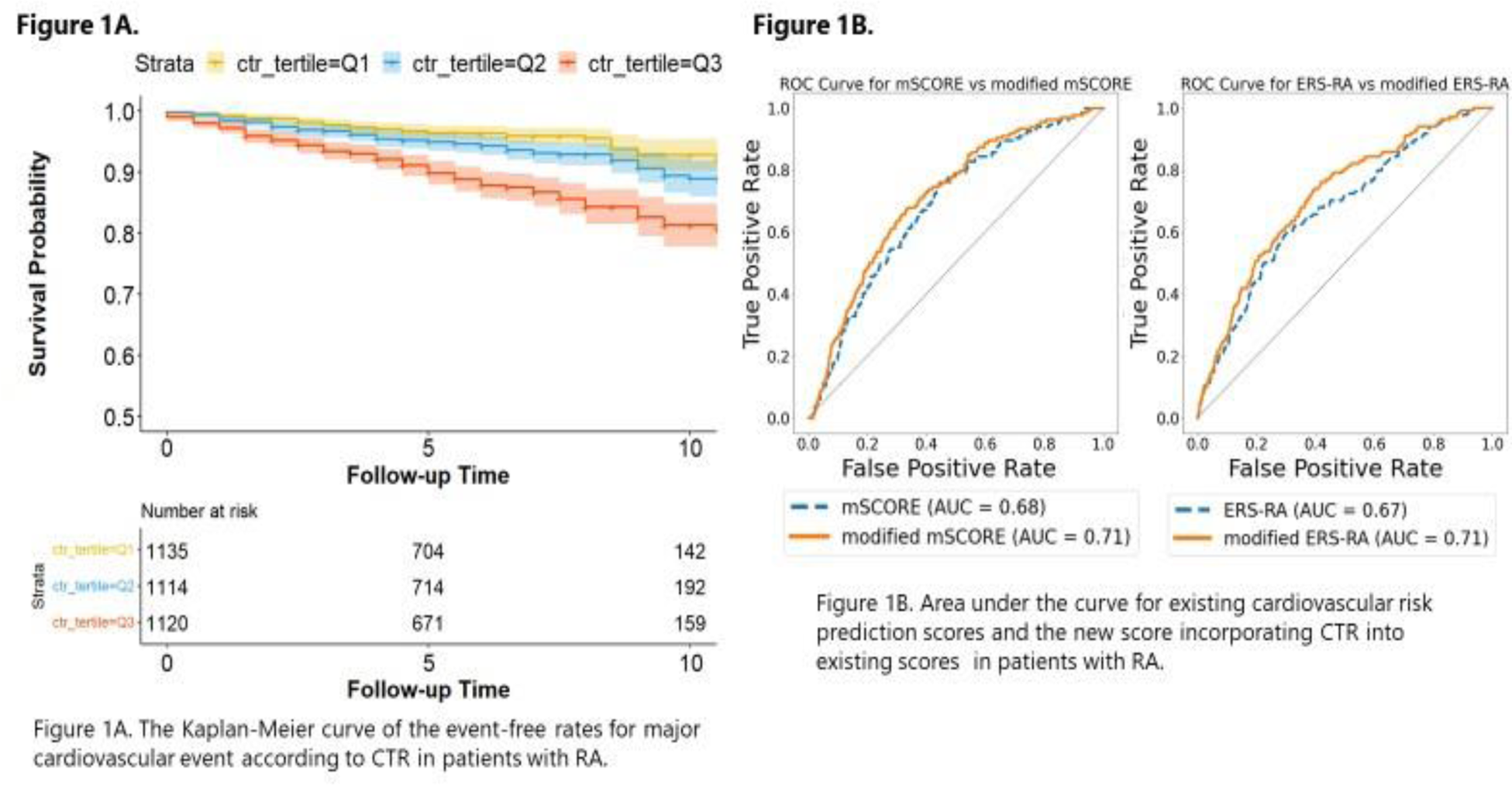
Results: Of a total of 3369 patients selected, with a mean age of 58.4 and 87.0% being female, 314 patients experienced MACE. In tests conducted on chest radiographs of 400 patients registered in the IORRA database, the average error for CTR was 0.025 (standard deviation [SD] ±0.9%), and for TBA −2.4 degrees (SD ±6.8 degrees). Only CTR was associated with MACE as an independent risk factor among the radiograph indices after adjusting for covariates (CTR, p <0.0001) (Table 1). Patients with higher CTR scores had lower MACE-free survival rates (P<0.0001) (Figure 1A). Based on the regression analysis using the Derivation cohort, additive percentages for each CTR tertile were +0, +0.3, +2.6 for mSCORE, and +0, +1.0, +8.7 for ERS-RA. In the Validation cohort, including CTR significantly improved the accuracy of mSCORE (AUC 0.685 vs. 0.713; p =0.006) and ERS-RA (AUC 0.675 vs. 0.714; p = 0.002) (Figure 1B).
Conclusion: The predictive accuracy for MACE in patients with RA is enhanced by incorporating CTR into the existing cardiovascular risk prediction scores.
REFERENCES: [1] Nakajima A, et al. Scand J Rheumatol. 2010; 39: 360-367.
[2] Ernest C, et al. Rheumatology. 2014; 53: 2143-2154.
[3] Honda S, et al. Rheumatology. 2023; 6: 2272-2283.
Acknowledgements: The authors would like to express sincere thanks to all the people involved with the IORRA cohort project.
Disclosure of Interests: Mayuko Fujisaki: None declared, Suguru Honda: None declared, Katsunori Ikari Asahi Kasei Pharma Co., Astellas Pharma Inc., AbbVie Japan GK, Ayumi Pharmaceutical Corporation, Bristol Myers Squibb Co. Ltd., Chugai Pharmaceutical Co., Ltd.
Eisai Co., Ltd., Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Kaken Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Co., Pfizer Japan Inc., Takeda Pharmaceutical Co. Ltd., Teijin Pharma Ltd, UCB Japan Co. Ltd., Ayumi Pharmaceutical Corp., Chugai Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd.
Nippon Kayaku Co., Ltd., Teijin Pharma Ltd., Eiichi Tanaka AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., GlaxoSmithKline K.K., Kyowa Pharma Chemical CO., Ltd., Janssen Pharmaceutical K.K., Mochida Pharmaceutical CO., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Teijin Pharma Ltd., Masayoshi Harigai AbbVie Japan GK, Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., GlaxoSmithKline K.K., Kissei Pharmaceutical Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Teijin Pharma Ltd., AbbVie, Boehringer-Ingelheim, Bristol Myers Squibb Co., Kissei Pharmaceutical Co., Ltd., Teijin Pharma., AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Bristol Myers Squibb Co., Chugai Pharmaceutical Co., Ltd, Daiichi-Sankyo, Inc., Eisai Co., Ltd, Kissei Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Co., Nippon Kayaku Co., Ltd., Sekisui Medical, Shionogi & Co., Ltd., Taisho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Teijin Pharma Ltd.