

Background: Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) are subtypes of antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV). In the phase 3 ADVOCATE trial in patients with GPA or MPA, avacopan was noninferior to a prednisone taper in achieving remission at week 26, and was superior in sustaining remission at week 52. 1 It was previously reported that 75 of 166 patients (45.2%) in the avacopan group and 69 of 164 patients (42.1%) in the prednisone taper group had ear, nose or throat (ENT) manifestations of active GPA/MPA. 1 Active AAV-ENT disease dropped to 1.2% (2 patients) at both weeks 26 and 52 in the avacopan group, and to 3.7% (6 patients) at week 26 and 3.0% (5 patients) at week 52 in the prednisone taper group. 2 However, the impact of avacopan on other outcomes in patients with ENT manifestations of GPA/MPA is unknown.
Objectives: To report the baseline characteristics, efficacy including remission and relapse rates, glucocorticoid (GC) toxicity, health-related quality of life (HRQoL), and safety of avacopan vs prednisone taper in a subgroup of patients in the ADVOCATE trial with ENT manifestations of GPA/MPA at baseline.
Methods: ADVOCATE was a double-blind, double-dummy, active-controlled trial, with patients randomized 1:1 to receive avacopan or a prednisone taper (60 mg/day tapered to 0 by week 21) on a background of cyclophosphamide (followed by azathioprine or mycophenolate mofetil) or rituximab. In this subgroup post hoc analysis of patients with ENT involvement at baseline based on Birmingham Vasculitis Activity Score (BVAS), key outcomes were the proportion of patients achieving overall remission at week 26 and sustaining remission at week 52. Other outcomes included proportion of patients with active ENT manifestations at all recorded timepoints, GC dose (prednisone-equivalent), Glucocorticoid Toxicity Index (GTI), HRQoL, and safety.
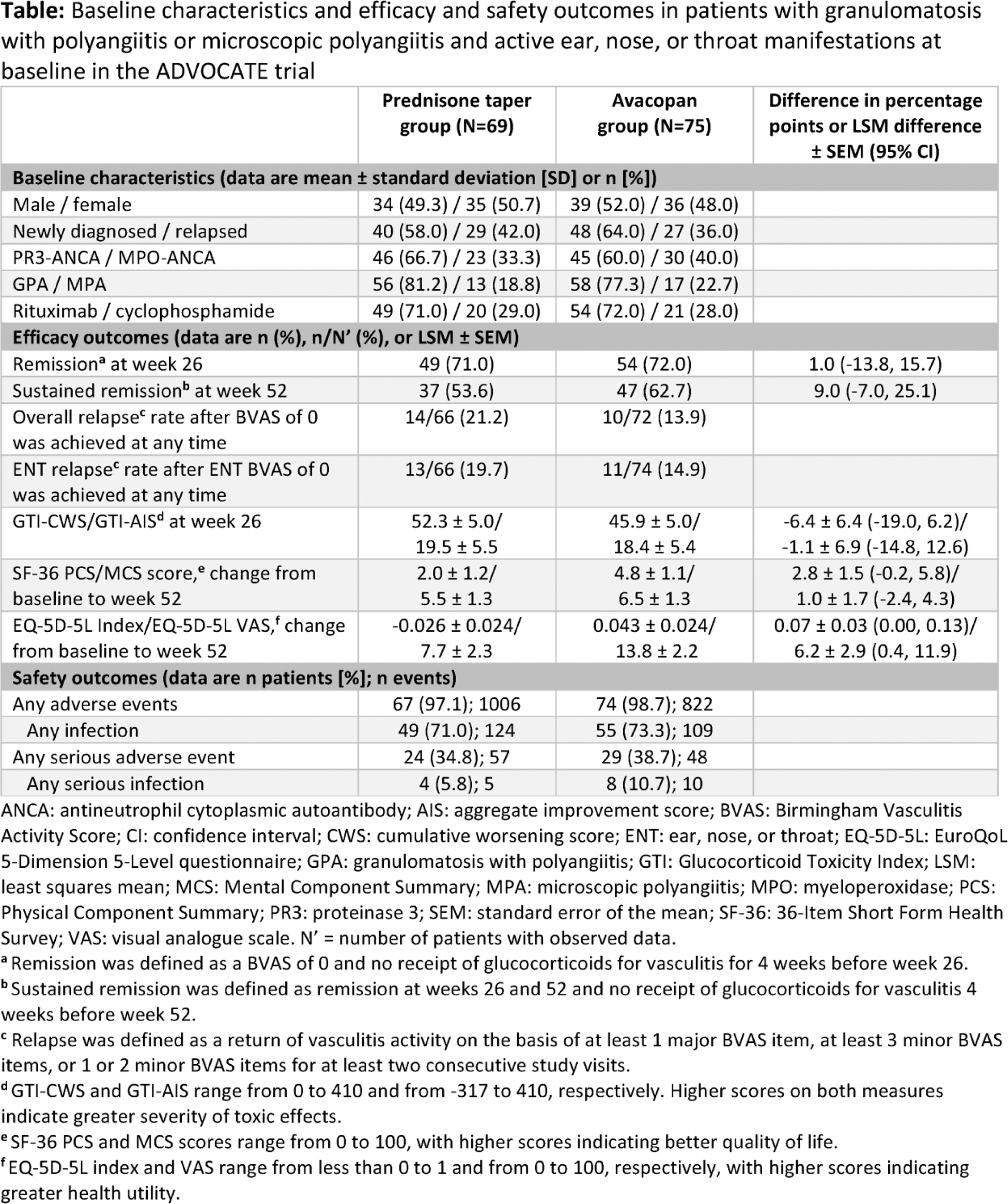
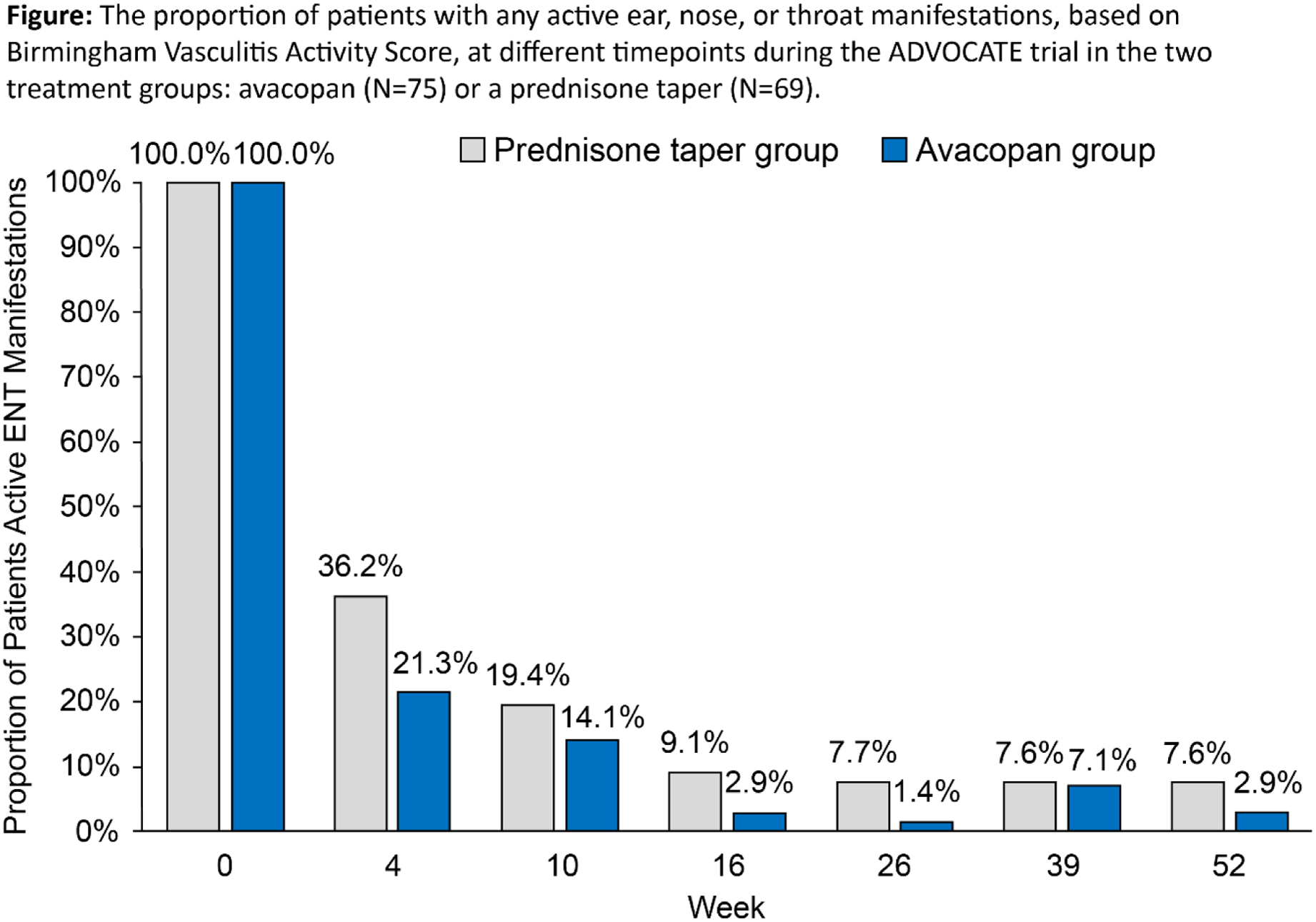
Results: Data from 144 patients (avacopan, N=75; prednisone taper, N=69) with active ENT manifestations at baseline were analyzed. Baseline characteristics were comparable across treatment groups ( Table 1 ). BVAS (mean ± SD) was 17.4 ± 7.1 and 17.0 ± 6.4 and age was 59.5 ± 15.4 and 56.5 ± 15.1 years in the avacopan and prednisone taper groups at baseline, respectively. The proportion of patients who had active ENT manifestations at week 4 decreased more rapidly with avacopan, dropping to 21.3%, vs 36.2% with prednisone taper ( Figure 1 ). The mean/median total all-source GC dose was 680/500 mg with avacopan vs 1677/1630 mg with prednisone taper after 4 weeks. A similar proportion of patients with avacopan vs prednisone taper achieved overall remission at week 26 (72.0% vs 71.0%; difference: 1.0%; 95% CI: -13.8%, 15.7%) and a higher proportion had sustained remission at week 52 (62.7% vs 53.6%; difference: 9.0%; 95% CI: -7.0%, 25.1%). Relapse within the ENT domain was numerically lower with avacopan (14.9%) vs prednisone taper (19.7%). Both GTI scores at week 26 were numerically lower (more favorable) with avacopan vs prednisone taper ( Table 1 ). The improvements from baseline in EuroQoL 5-Dimension 5-Level index and visual analogue scale were greater with avacopan vs prednisone taper at week 52 ( Table 1 ). Infections occurred in 55 patients (73.3%) in the avacopan group (109 events) and in 49 patients (71.0%) in the prednisone taper group (124 events); 1 death (worsening of vasculitis) occurred in the avacopan group (1.3%).
Conclusion: Findings from this post hoc subgroup analysis of ADVOCATE in patients with MPA/GPA and active ENT manifestations at baseline showed that avacopan was associated with faster resolution of ENT manifestations, higher rate of overall sustained remission, lower relapse rate, numerically lower GC-related toxicity, greater improvement in HRQoL from baseline, and similar safety vs a prednisone taper. These data indicate avacopan may be efficacious in patients with ENT involvement of GPA/MPA.
REFERENCES: [1] Jayne DRW et al. N Engl J Med 2021;384:599-609; 2. Specks U et al. Am J Respir Crit Care Med 2022;205:A4780.
Acknowledgements: The authors would like to thank the patients, personnel, and investigators at ADVOCATE sites throughout the world. We also thank Cactus Life Sciences (part of Cactus Communications), Mumbai, India, for their editorial support.
Disclosure of Interests: Robert Spiera AbbVie/Abbott (Consultant); Amgen, (Consultant); GSK (Consultant); Novartis (Consultant); Sanofi (Consultant), AbbVie/Abbott (Grant/research support); AstraZeneca (Grant/research support); ChemoCentryx (Grant/research support); Corbus (Grant/research support); Formation Biologics (Grant/research support); GSK (Grant/research support); InflaRx (Grant/research support); Kadmon (Grant/research support); Novartis (Grant/research support); Principia (Grant/research support), Robert Lebovics: None declared, Sarah Bray Amgen (Employee), Rachel E. Gurlin Amgen (Employee), David R. W. Jayne Amgen (Speakers bureau); GSK (Speakers bureau); CSL Vifor (Speakers bureau), Alentis (Shareholder); Aurinia (Shareholder), AstraZeneca (Consultant); Boehringer Ingelheim (Consultant); Chinook (Consultant); CSL Vifor (Consultant); GSK (Consultant); Novartis (Consultant); Roche (Consultant), GSK (Grant/research support); Roche (Grant/research support); CSL Vifor (Grant/research support), Peter A. Merkel Kyverna (Stock options), Amgen (Consultant); Argenx (Consultant); AstraZeneca (Consultant); Boehringer Ingelheim (Consultant); Bristol Myers Squibb (Consultant); Cabaletta (Consultant); CSL Behring (Consultant); GSK (Consultant); HiBio (Consultant); InflaRx (Consultant); Janssen (Consultant); Jubilant (Consultant); Kyverna (Consultant) MiroBio (Consultant); Novartis (Consultant); NS Pharma (Consultant); Q32 (Consultant); Regeneron (Consultant); Sanofi (Consultant); Sparrow (Consultant); Takeda (Consultant); UpToDate (Royalties); Visterra (Consultant), AbbVie/Abbott (Grant/research support); Amgen (Grant/research support); AstraZeneca (Grant/research support); Boehringer Ingelheim (Grant/research support); Bristol Myers Squibb (Grant/research support); Eicos (Grant/research support); Electra (Grant/research support); Genentech (Grant/research support); GSK (Grant/research support); InflaRx (Grant/research support); Neutrolis (Grant/research support); Takeda (Grant/research support).