

Background: Lupus nephritis (LN) leads to end-stage kidney disease (ESKD) in >20% of patients despite optimal treatment. Up to 30% of LN patients have membranous LN which is characterized by subepithelial immune deposits without immune infiltration in the glomeruli. Despite its association with ESKD, membranous LN is considered a milder type of LN with no consensus on the optimal use of immunosuppression.
Objectives: To develop mechanistic hypotheses of disease, we analyzed kidney samples from patients with LN using a whole slide spatially resolved proteomic approach as part of the Accelerating Medicines Partnership in RA/SLE. We report here the initial analysis.
Methods: We developed a serial immunohistochemistry (sIHC) staining workflow to stain for 18 antibodies, DNA, and PAS to be visualized on a single section via a cycle of staining, imaging, and destaining. This included incubation of FFPE slides with primary antibody, secondary HRP reagents, AEC-Red Chromogen, and Hematoxylin. Image processing was performed using HALO (Indica Labs) and included deconvolution of single-color channels, registration, fusion, cell segmentation, and automated tissue classification. To minimize batch effect, the analytical pipeline included within sample CLR-normalization and scaling, followed by harmonization (Harmony).
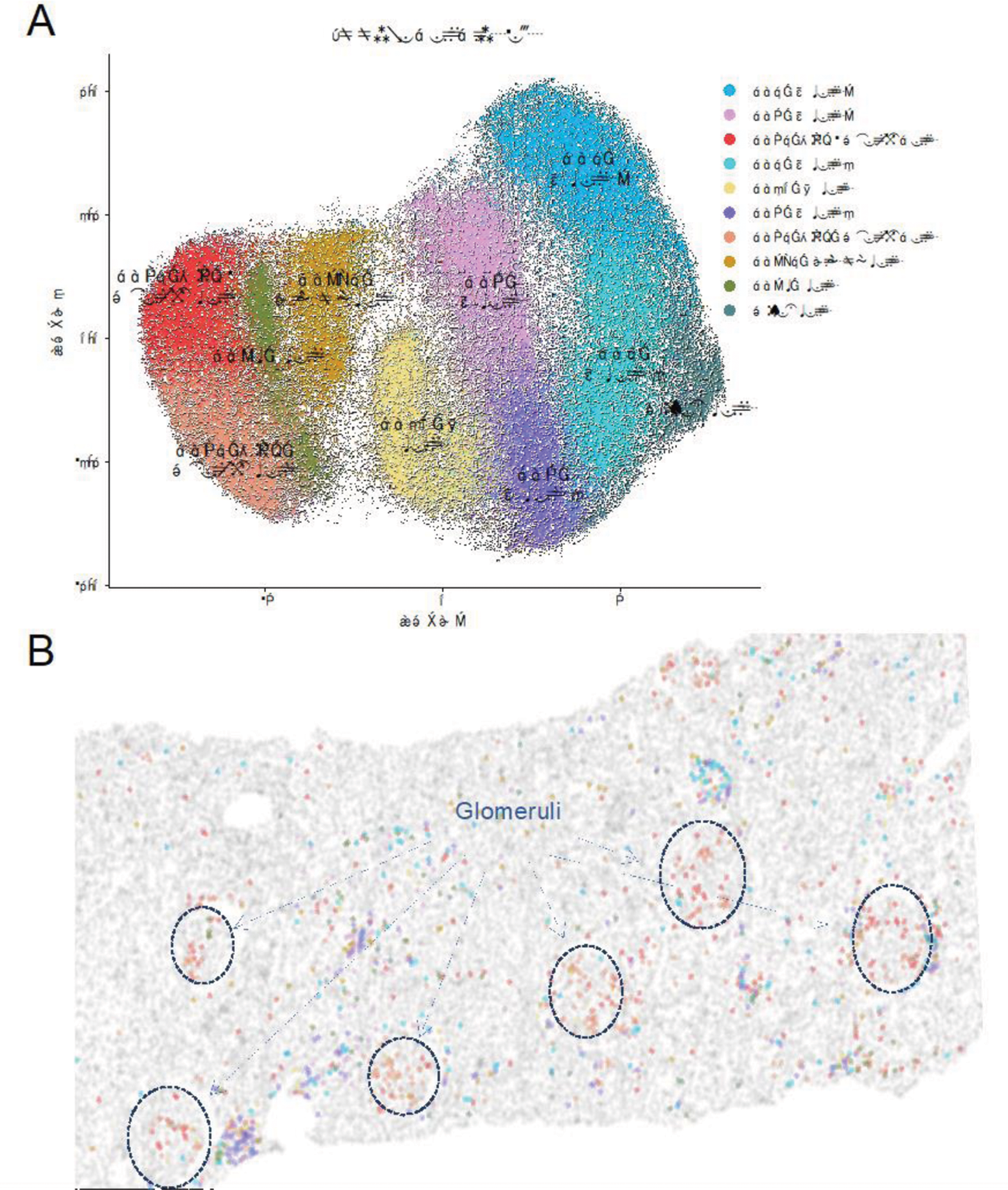
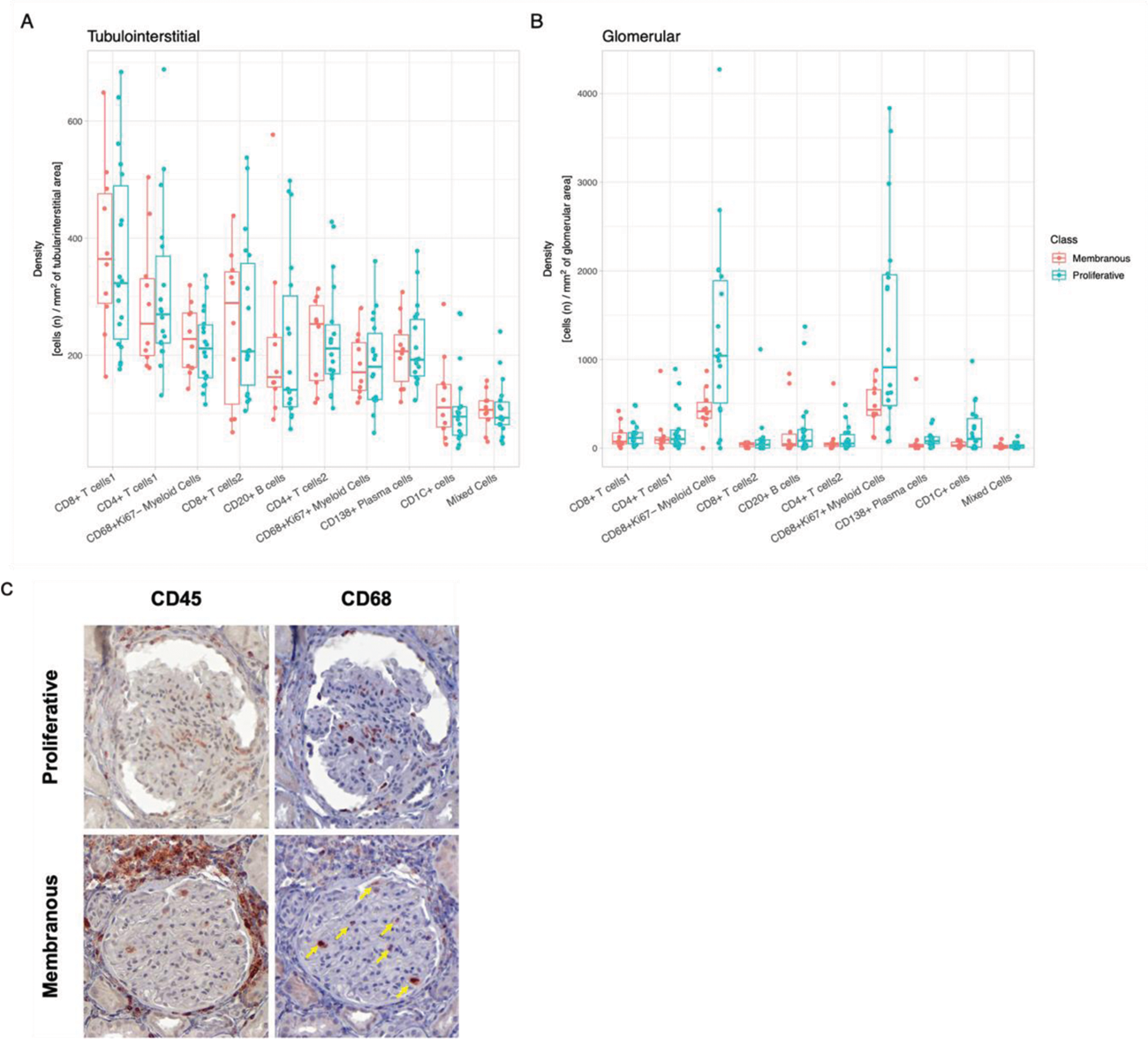
Results: In this initial analysis, we included 29 clinically indicated kidney biopsies classified as LN: 13 pure proliferative (ISN class III or IV), 10 pure membranous (ISN class V), 5 mixed, and 1 ISN class II). Patients were 79% female, 34% White, 31% Black, 10% Asian, and 24% identified with Other race/ethnicity. We detected 182,783 CD45+ cells out of 1,913,845 cell objects. Our analysis identified 10 immune cell clusters at low resolution (Figure 1A). Figure 1B displays the tissue distribution of each cell subset. B and T lymphocytes dominated the tubulointerstitium. The CD68+ myeloid subsets were the predominant cell type in the glomeruli (Figure 2). More than half of CD68+ cells expressed Ki67 indicating active proliferation. Surprisingly, we identified intraglomerular CD68+ cells (including endocapillary) also in patients with pure membranous LN, but at a lower tissue density than proliferative LN (Figure 2B). Figure 2C demonstrates intraglomerular CD68+ cells in a biopsy classified as pure membranous LN by two experienced renal pathologists.
Conclusion: sIHC can be successfully employed to perform multiplexed whole slide analysis harnessing both the subcellular resolution (brightfield) and the reliability of IHC. Our analysis revealed intraglomerular CD68+ myeloid cells in pure membranous LN. By traditional clinical pathology, intraglomerular/ endocapillary immune cells characterize proliferative LN and are not consistent with pure membranous LN. These findings implicate macrophages/monocytes in the glomerular disease in membranous LN with therapeutic implications. The analysis of 90 additional biopsies and a myeloid-focused panel is underway to validate and extend these findings.
Phenotype and spatial distribution of intrarenal immune cells. (A) UMAP of CD45+ cells (n=182,783, 29 patients) indicating the low-resolution cluster annotation. (B) Digital reproduction of a representative biopsy displaying the distribution of the cells clusters (colors matching panel A). ISN class III, NIH Activity Index 6, NIH Chronicity Index 3.
Myeloid cells dominate intraglomerular inflammation, including membranous LN. Box plots displaying the density (cells/ area) of the immune cell clusters in the tubulointerstitium (A) and glomeruli (B) according to ISN class. CD68+ myeloid cell clusters showed a statistically significant higher intraglomerular density compared to all other clusters in proliferative and membranous LN (p<0.05, Wilcoxon). Proliferative LN (n=18); membranous LN (n=10). (C) Immunohistochemistry images displaying the expression of CD45 and CD68 in a glomerulus from a patient with proliferative LN and one with pure membranous LN. The yellow arrows indicate intraglomerular CD68+ cells in membranous LN.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Andrea Fava Sanofi, AnnexonBio, AstraZeneca, UCB., Chen-Yu Lee: None declared, Matthew Caleb Marlin: None declared, Xiaoping Yang: None declared, Tayte Stephens: None declared, Alessandra Ida Celia: None declared, Jeffrey Hodgin AstraZeneca, Eli Lilly, Gilead, Janssen, Moderna, Novo Nordisk, Regeneron, Dawit Demeke: None declared, Peter Izmirly: None declared, Jill Buyon BMS, GSK, Related Sciences, Ventus, Artiva, Equillium, Chaim Putterman Equillium, KidneyCure, Progentec, Judith A. James Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Novartis, Progentec Biosciences., Michelle Petri Arthros-FocusMedEd, Aurinia, Amgen, AnaptysBio, Annexon Bio, Argenx, AstraZeneca, Axdev, Boxer Capital, Cabaletto Bio, Caribou Biosciences Inc, CVS Health, Escient Pharmaceuticals, Exo Therapeutics, Gentibio, GSK, Horizon Therapeutics, iCell Gene Therapeutics, Idorsia Pharmaceuticals, Kira Pharmaceuticals, Eli Lilly, MedShr, Momenta Pharmaceuticals, Nexstone Immunology, Nimbus Lakshmi, Proviant, Regeneron Pharmaceuticals, Sanofi, Seismic Therapeutic, Sinomab Biosciences, Takeda, Tenet Medicines Inc, TG Therapeutics, UCB, Zydus. DSMB: CTI Clinical Trial and Consulting Services, Emergent Biosolutions, IQVIA, Merck EMD Serono., Aurinia, Eli Lilly, Exagen, GSK, Janssen, AstraZeneca, Joel M Guthridge: None declared, Avi Rosenberg: None declared.