

Background: The pathogenesis of synovitis in systemic sclerosis (SSc) is unknown. Due to the lack of evidence-based therapies, SSc patients with synovitis are treated as rheumatoid arthritis (RA) patients, however with unknown effect. Previously, we showed that SSc synovitis differs from RA synovitis with a prevalent pauci-immune pathotype.
Objectives: To decipher the histological, cellular, and transcriptional differences between SSc and RA synovitis, specifically in synovial fibroblasts (SF).
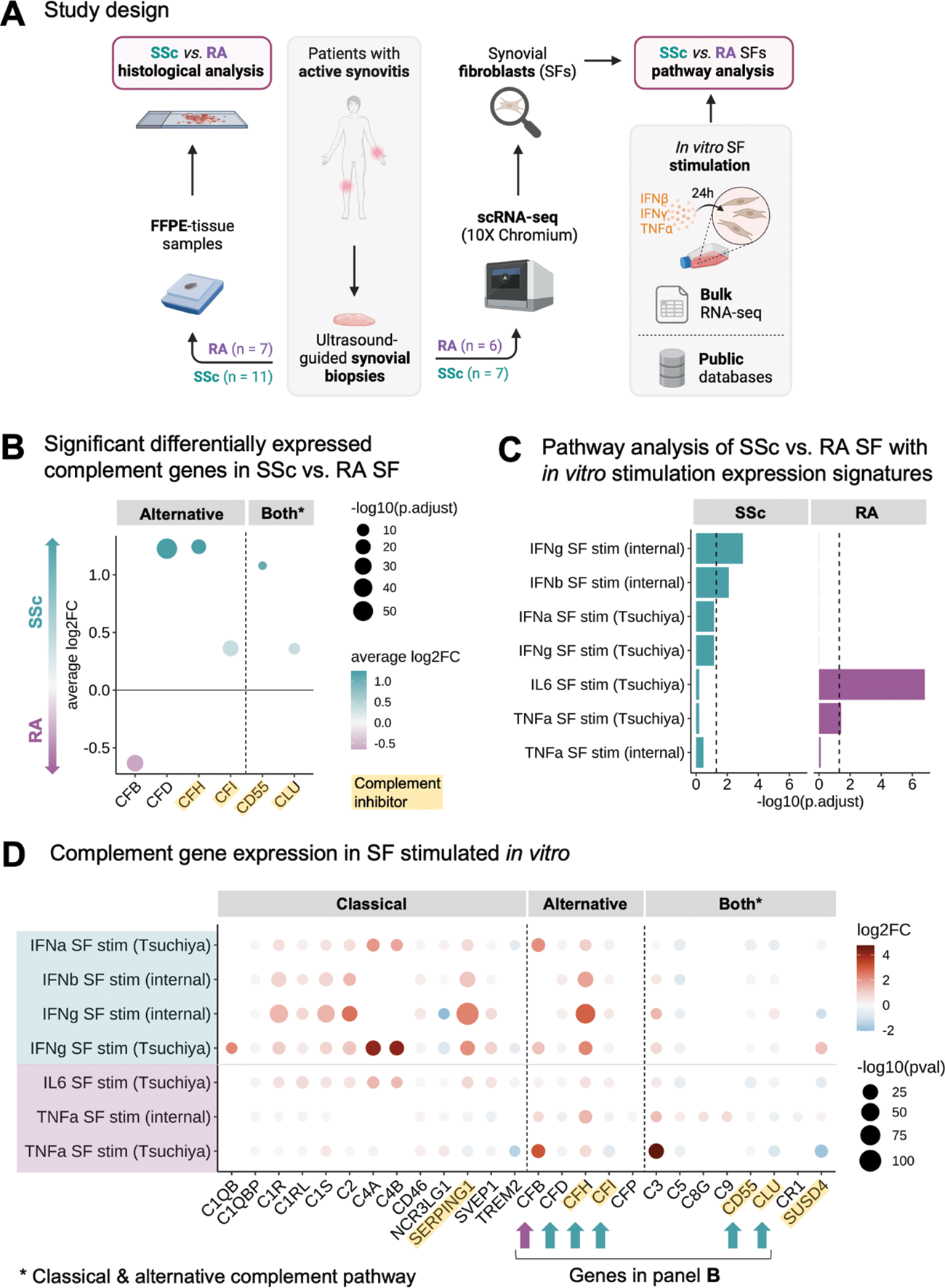
Methods: We collected ultrasound-guided synovial biopsies from 11 SSc and 7 RA patients. All samples were analysed histologically, including Krenn synovitis score (0-9) and pathotype characterisation. Additionally, 7 SSc and 6 RA samples were dissociated and processed for single-cell RNA-sequencing (20,705 cells in SSc and 31,681 in RA). Due to the pauci-immune nature of the synovitis, we focused our analysis on the SF population (4,638 SF in SSc, 6,097 in RA). We performed differential gene expression analysis, over-representation pathway analyses and calculated signature expression scores between SSc and RA SF. Pathway analyses included publicly available datasets and bulk RNA sequencing data from SF stimulated in vitro with TNF-α, IFN-α/b, IFN-γ, and IL-6 from internal experiments and published data 1 (Figure 1A).
Results: The Krenn synovitis score was lower in SSc than in RA (2.1 ± 1.4 vs. 4.2 ± 1.0, p = 0.01). There was no difference in fibrosis (Elastica van Gieson staining) and vascularisation (CD31 staining) between SSc and RA synovitis. We confirmed a pauci-immune pathotype characteristic of SSc synovitis (91% vs. 43% of RA biopsies, p = 0.04). Consistently, neutrophils were more prevalent in RA as compared to SSc synovitis (p < 0.01). On the single-cell level, we identified seven SF cell states defined by the expression of the marker genes PRG4 , CHI3L2 , COMP , CXCL12 , CXCL14 , MFAP5 , and POSTN consistent with the current literature in RA 2 . The proportion of SF clusters differed between both diseases with a higher enrichment of MFAP5 + (Fold-change (FC) = 3.81, false discovery rate (FDR) < 0.001) and COMP + SF in SSc (FC = 2.28, FDR < 0.001), and POSTN + (FC = 3.12, FDR < 0.001) and CXCL12 + SF in RA (FC = 1.70, FDR < 0.001). We identified 675 significant differentially expressed genes (FDR < 0.01, |log 2 FC| > 0.25) and several differentially activated signalling pathways between SSc and RA SF. In particular, SSc SF were enriched in IFN-α and IFN-γ (both FDR < 0.018) and complement and coagulation pathways (FDR < 0.008), especially in genes of the alternative complement pathway (Figure 1B). RA SF showed a strong enrichment in TNF-α signaling (FDR < 6.3e-12) and inflammatory response genes (FDR < 0.018). Using the transcriptional signature of in vitro stimulated SF as gene sets, we confirmed the IFN response in SSc SF, whereas TNF-α and IL-6 response signatures were more dominant in RA SF (Figure 1C). Interestingly, IFN-α/β and IFN-γ stimulation of SF in vitro led to an activation pattern of alternative complement pathway genes similar to that observed in SSc SF (Figure 1D). IFN and complement signature scores were significantly higher in sublining CXCL12 + SF in SSc compared to RA (both p < 2.22e-16). To identify the drivers of the IFN signature in SSc SF, we assessed the expression of the different IFN genes in synovial cell populations. Only IFNG was expressed, mainly by T cells, which is consistent with the preferential expression of IFN response genes in the sublining synovium.
Conclusion: This first in-depth characterization of the SSc synovium revealed hallmark tissue, cellular and molecular changes between SSc and RA synovitis. We found a strong enrichment of the IFN signalling pathway in SSc SF, possibly leading to a dysregulation of the alternative complement pathway. Our findings provide the basis for the development of specific targeted therapies for synovitis in SSc.
REFERENCES: [1] Tsuchiya, Haruka, et al. Annals of the Rheumatic Diseases 80.4 (2021): 440-450.
[2] Zhang, Fan, et al. Nature (2023): 1-9.
Abbreviations: log2FC = log 2 fold-change, RA = rheumatoid arthritis, SF = synovial fibroblasts, SSc = systemic sclerosis, stim = stimulation. Panel A created with BioRender.
Acknowledgements: This project is funded by FOREUM – Foundation for Research in Rheumatology. We thank our patient research partners Joëlle Messmer & Andreas Eisenring.
Disclosure of Interests: Celina Geiss: None declared, Miranda Houtman: None declared, Raphael Micheroli: None declared, Yannis Djeffal: None declared, Mojca Frank Bertoncelj: None declared, Sam G. Edalat: None declared, Kristina Buerki: None declared, Chantal Pauli: None declared, Michael Bonelli GSK, Galapagos, Thomas Karonitsch: None declared, Oliver Distler 4P-Pharma, Abbvie, Acceleron, Alcimed, Altavant, Amgen, AnaMar, Argenx, Arxx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, Merck, Miltenyi Biotec, Mitsubishi Tanabe, Novartis, Orion, Prometheus, Redxpharma, Roivant, Topadur and UCB, Co-founder of CITUS AG, 4P-Pharma, Abbvie, Acceleron, Alcimed, Altavant, Amgen, AnaMar, Argenx, Arxx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, Merck, Miltenyi Biotec, Mitsubishi Tanabe, Novartis, Orion, Prometheus, Redxpharma, Roivant, Topadur and UCB, 4P-Pharma, Abbvie, Acceleron, Alcimed, Altavant, Amgen, AnaMar, Argenx, Arxx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, Merck, Miltenyi Biotec, Mitsubishi Tanabe, Novartis, Orion, Prometheus, Redxpharma, Roivant, Topadur and UCB, Caroline Ospelt: None declared, Muriel Elhai: None declared.