

Background: Knee Osteoarthritis (KOA) is a leading cause of disability with nearly a quarter of adults over 40 affected worldwide. Despite its widespread impact, no approved pharmacological solution exists to halt, slow, or reverse the progression of KOA. One of the main challenges in the KOA research aiming to find a cure is using total knee replacement (TKR) as an outcome, as many regulatory agencies rely on it to show the improvements in progression in knee osteoarthritis (KOA). However, the decision to undergo TKR is influenced by various factors beyond disease progression, such as education, income, and health insurance. To address these limitations, we recently validated a new outcome, namely, end-stage KOA (esKOA). A knee was classified as having esKOA if it met either of the following criteria: 1) Displaying moderate to severe KOA symptoms (defined as a combined WOMAC pain and disability score of 12 or above) in conjunction with the most severe radiographic KOA (i.e., KL grade = 4, the maximum KL grade); 2) Exhibiting intense KOA symptoms (a combined WOMAC pain and disability score of 23 or more) alongside persistent knee pain and either mild or moderate radiographic KOA (i.e., KL grade = 2 or 3). Unlike TKR incidence, esKOA is an endpoint that represents the advanced stage of KOA, independent of external factors influencing TKR decisions. Furthermore, an esKOA and change in esKOA predict the subsequent occurrence of TKR. Given the lack of a cure, tools that can predict the progression of KOA would be invaluable. Predictive tools can enhance the efficacy of clinical trials by identifying appropriate candidates and ensuring that participants are likely to show disease progression during the trial period.
Objectives: To enhance future trials that use esKOA as an outcome, our study focuses on developing and validating a machine-learning tool to identify individuals likely to develop esKOA within 2 to 5 years. We also aim to implement an online tool delivering the prediction of esKOA for 2 to 5 years based on the developed machine learning algorithm.
Methods: Using the Osteoarthritis Initiative (OAI) data, we trained the models with 3,259 participants and validated them with 616 participants. The Multicenter Osteoarthritis Study (MOST) data, consisting of 1,795participants, was employed for external validation. Our primary outcome was predicting the onset of esKOA at 2-to-2.5 years and 4-to-5 years. Our analysis considered 40 candidate predictors, including demographics, clinical history, physical examination, and X-ray evaluations. We also evaluated the models with top nine predictors among the 40 predictors. Using the models with nine predictors, we developed an online tool that predicts the probability of progression to esKOA at 2-to-2.5 years and 4-to-5 years.
Results: The Area Under Curve (AUC) obtained from external validation (i.e., MOST) using 40 predictors at 2.5 years was 0.861 (95% CI 0.832 to 0.886), and at 5 years was 0.854 (95% CI 0.830 to 0.877). The models with nine predictors showed comparable performance (Figure 1). Using the nine predictors, we developed an online tool that can be found at the following link:
Receiver operating characteristic (ROC) curves showing the performance of models for prediction of esKOA at 2-to-2.5 years and 4-to-5 years, using nine predictors. A) Validation Dataset (OAI) at 2 years. B) Validation Dataset (OAI) at 4 years. C) External Validation Dataset (MOST) at 2.5 years. D) External Validation Dataset (MOST) at 5 years. Red Points show the corresponding True Positive Rate (TPR) and False Positive Rate (FPR) for the selected thresholds. AUC: Area Under Curve; MOST: Multicenter Osteoarthritis Study; OAI: Osteoarthritis Initiative.
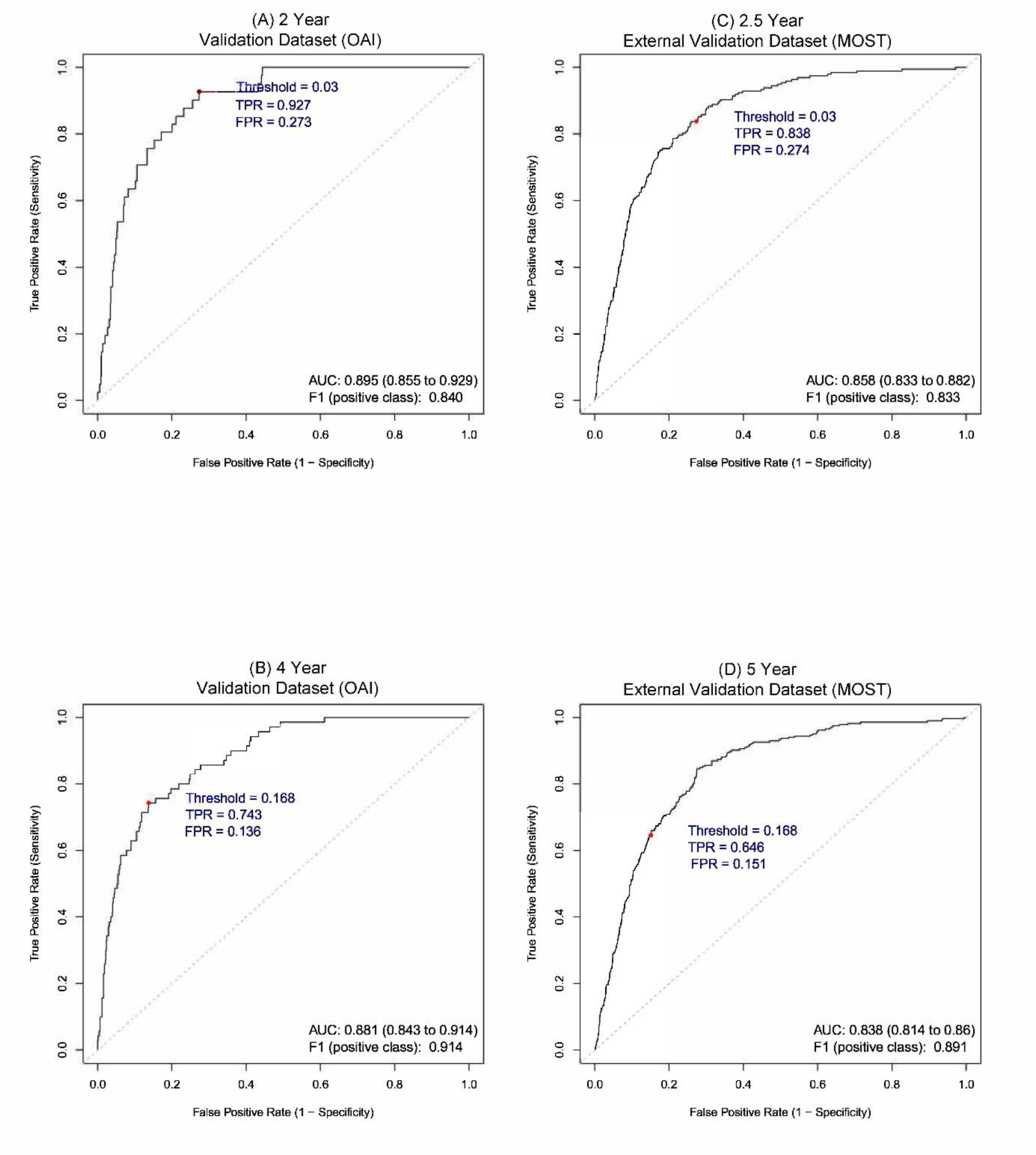
Conclusion: Our study unveils a robust, externally validated machine learning tool proficient in predicting the onset of esKOA over the next 2 to 5 years. Our screening tool can lead to more efficient KOA trials.
REFERENCES: NIL.
Acknowledgements: We acknowledge the provision of datasets and/or research tools from two cohort studies: the Osteoarthritis Initiative (OAI) and the Multicenter Osteoarthritis Study (MOST). The OAI is a collaborative informatics system created by the National Institute of Mental Health and the National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) to provide a worldwide resource to quicken the pace of biomarker identification, scientific investigation and osteoarthritis drug development. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation; GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using OAI public-use data sets and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. The OAI data repository is housed within the National Institute of Mental Health (NIMH) Data Archive (NDA). For MOST, we wish to acknowledge the contributions of the study participants, investigators and research staff involved. MOST is comprised of four (4) cooperative grants: U01 AG18820 to David T Felson (Boston University); U01 AG18832 to James Torner (University of Iowa); U01 AG18947 to Cora E Lewis (University of Alabama at Birmingham); and U01 AG19069 to Michael C Nevitt (University of California, San Francisco), funded by the National Institutes of Health (NIH), a branch of the Department of Health and Human Services, and conducted by MOST investigators. This manuscript was prepared using MOST data and does not claim, infer, or imply endorsement by MOST, by the MOST investigators and their respective institutions, or by the University of California, of the Data Recipients’ (i.e., our) use of the Data, of the entity or personnel conducting the research (i.e., for this paper), or of any results of the research (i.e., of the current study).
Disclosure of Interests: Zubeyir Salis: None declared, Jeffrey Driban A consultant for Pfizer Inc. and Eli Lilly and Company and served on an advisory board for Novartis, Timothy McAlindon A consultant for Remedium-Bio, Anika, Chemocentryx, Grunenthal, Kolon Tissue Gene, Novartis, BioSplice, Organogenesis, and Pfizer Inc.