

Background: Cytokines including IL-17A, IL-17F and TNFα are thought to be central to spondyloarthropathy (SpA) and psoriasis immunopathology. Although IL-17A and IL-17F show strong structural homology and overlapping functions, the combined blockade of IL-17A and IL-17F is more effective in psoriasis. IL-23 is thought to be a critical regulator of IL-17 in a subset of T-helper cells. Our previous work demonstrates both IL-23 dependent and independent IL-17A and IL-17F production. In this study we sought to determine the pathways involved in production of IL-17 at site of disease. Enthesitis is the cardinal process in Spa, however, even the most rudimentary biology of IL-17F at the human enthesis remains undefined.
Objectives: The aim of the study was to characterise the IL-23 dependent and independent production of IL-17F at the normal enthesis from innate and adaptive T-cells. To determine impact of IL-17F on stromal function including chemokine production and in vitro spine entheseal mesenchymal stem cell (MSC) osteogenesis.
Methods: Spinal enthesis samples were collected from spinal decompression/scoliosis correction surgery. Immune cell populations were isolated by mechanical digest followed by red cell lysis. T-cells were stimulated with plate bound anti-CD3 (100ng/ml) and soluble anti-CD28 (100ng/ml) for 72hrs in RPMI media with GolgiPlug addition prior to intracellular staining of IL-17A and IL-17F. Samples were analysed either by flow cytometry or CyTOF analysis using a 36-marker in-depth immune cell phenotyping CyTOF panel (n=5). Similar stimulations with/without IL-23 (50ng/ml) were undertaken and a multiplex bead immunoassay measured inflammatory cytokine production (n=4). Mesenchymal stem cells (MSCs) were isolated from entheseal soft tissue and bone using a collagenase digest and placed in an osteogenic differentiation system in the presence of IL-17A (50ng/ml), IL-17F (50ng/ml) or both (n=5). The effects on osteogenesis were evaluated by alkaline phosphatase and calcium levels along with a 48-gene panel.
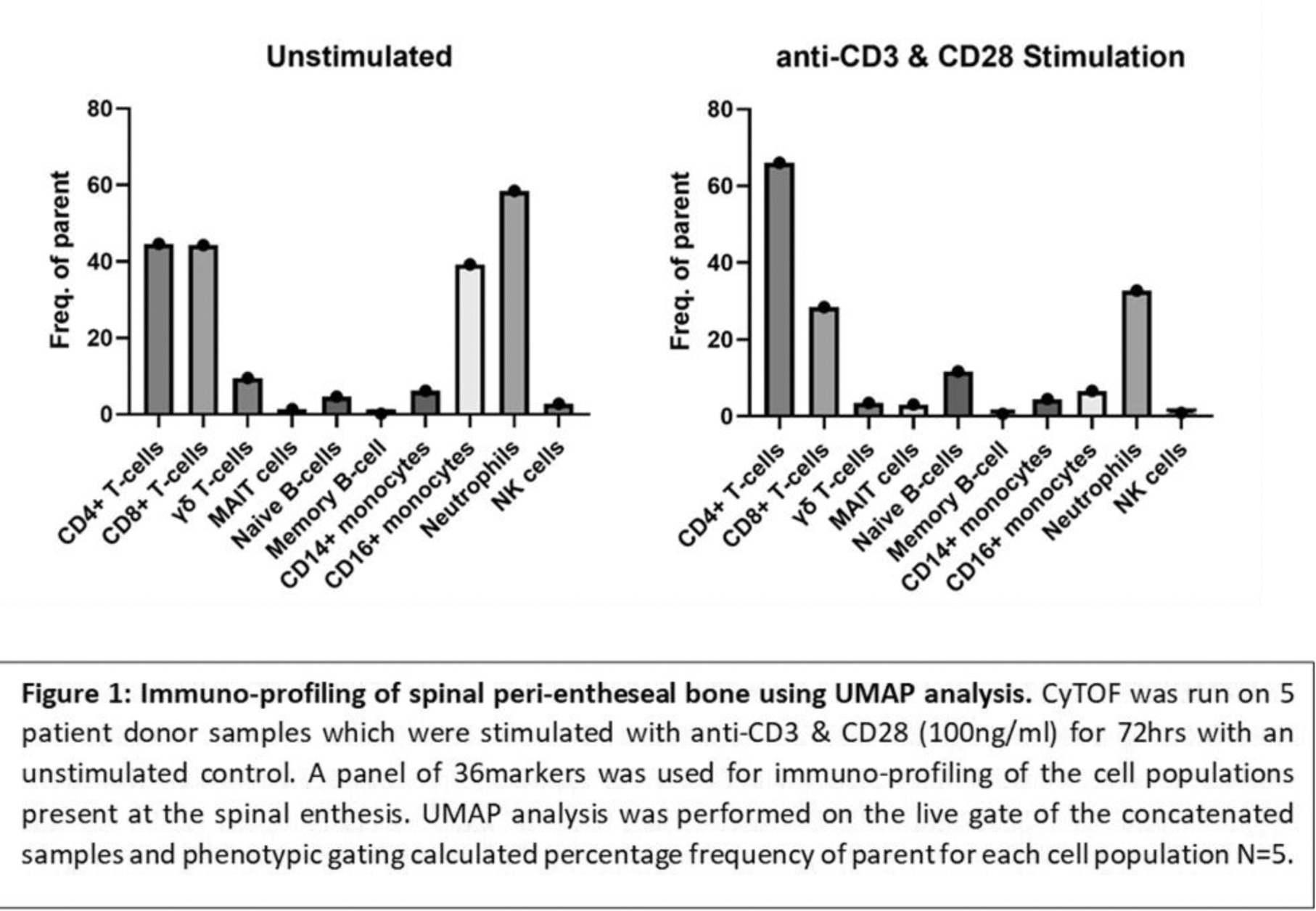
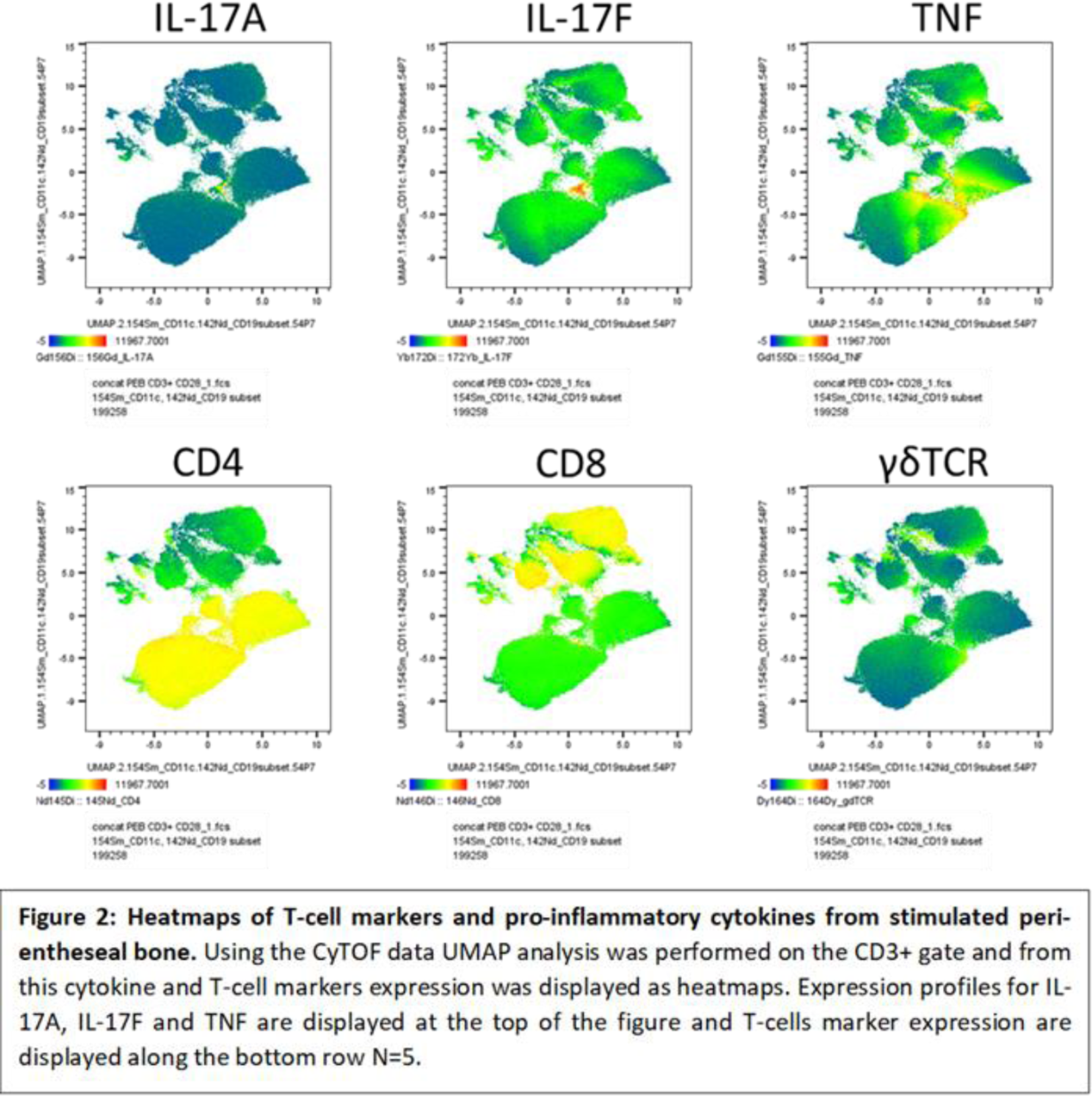
Results: Following aCD3/aCD28 stimulation both conventional flow and CyTOF demonstrated the inducible expression of both IL-17A and IL-17F by innate and adaptive T cells predominantly from the CD4 T-cells and γδ T-cells. After 72hrs of T-cell activation, there are higher levels of IL-17F than IL-17A at the enthesis. Addition of IL-23 to aCD3/aCD28 stimulated cells enhanced IL-17A/F production but IL-23 was unable to stimulate expression alone. Culture expanded entheseal MSCs exposed to IL-17A and IL-17F or both, showed IL-17A and IL-17F marginally increased alkaline phosphatase but was not associated with significant changes in MSC osteogenesis or osteogenic transcripts such as RUNX, BGLAP or RANKL. With respect to enthesis stromal function, IL-17A or IL-17F in combination with TNF dramatically upregulated CCL-20. However, IL-17A and IL-17F together show no additive effects.
Conclusion: This is the first functional study of IL-17F immunobiology of the human enthesis. Akin to the normal skin the normal enthesis has innate and adaptive T-cells capable of induced IL-17F expression in addition to IL-17A. Addition of IL-23 to activated T-cells enhanced IL-17F but IL-23 driven production of IL-17F expression was not observed. We noted the synergistic effects of IL-17F with TNF on stromal function but not on MSC osteogenesis activity from entheseal derived cells.
REFERENCES: NIL.
Acknowledgements: This work is funded in collaboration with UCB Pharma who provided support and access for CyTOF work.
Disclosure of Interests: Nicole McDermott: None declared, Mark Harland: None declared, Chi Wong: None declared, Ala Altaie: None declared, Tom Macleod: None declared, Charlie Bridgewood: None declared, Peter Loughenbury: None declared, Robert Dunsmuir: None declared, Almas Khan: None declared, Abhay S Rao: None declared, Vishal Borse: None declared, Avneet Manghera UCB, Stevan Shaw UCB, Dennis McGonagle UCB, Abbvie, BMS, Janssen Pharmaceuticals.