

Background: Immune cell heterogeneity in rheumatoid arthritis (RA) is well recognised, with the emergence of novel synovial tissue cellular phenotypes. However, the need for easily accessible blood-based biomarkers remains and the relevance of cellular heterogeneity across the RA continuum has not been fully defined.
Objectives: To investigate the diversity and functional states of immune cell lineages in the peripheral blood in early and established stages of RA.
Methods: Peripheral blood mononuclear cells (PBMCs) were collected from 82 patients with RA comprising 30 treatment-naïve early RA (ERA; symptom duration ≤12 months) and 52 established RA (estRA; symptom duration > 12 months) and 19 healthy individuals. 23/52 (44%) estRA who failed ≥ 2 classes of b and/or tsDMARDs were classified as difficult-to-treat RA (D2T-RA).[1] Using a panel of 35 antibodies, mass cytometry time-of-flight (CyTOF) was performed on the PBMCs. Unsupervised clustering and linear mixed-effects models were applied to compare healthy and RA patients. Further analyses were performed to compare across RA subgroups (ERA versus non-D2T estRA and ERA versus D2T-RA). All analyses were adjusted for age, sex, seropositivity and batch effects and performed using R v4.0.2. A false discovery rate of 0.05 was applied.
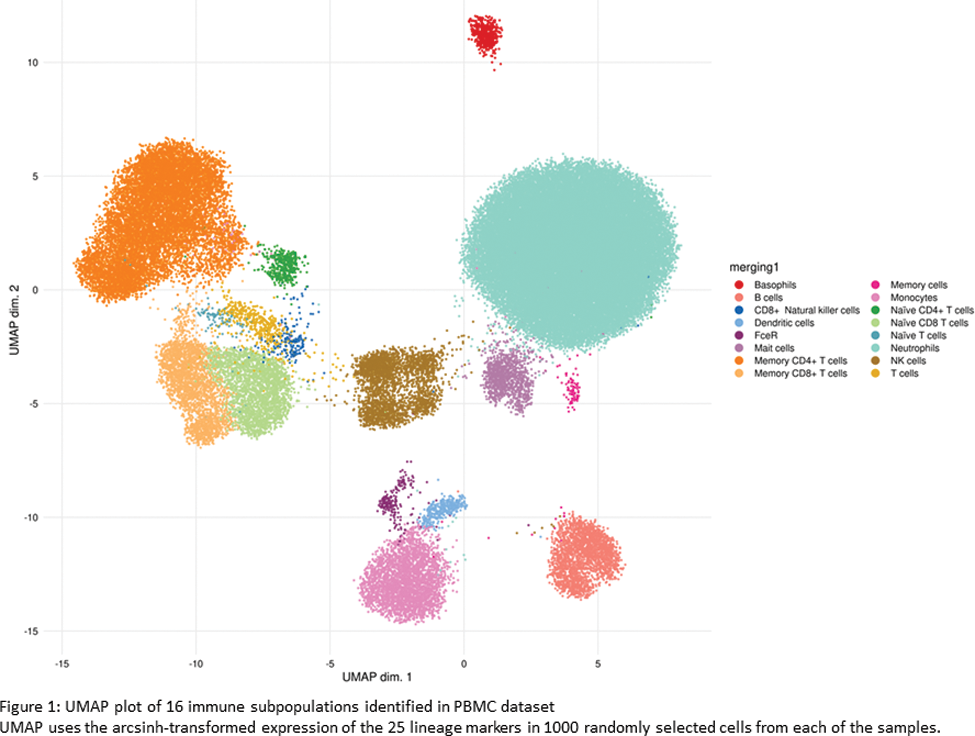
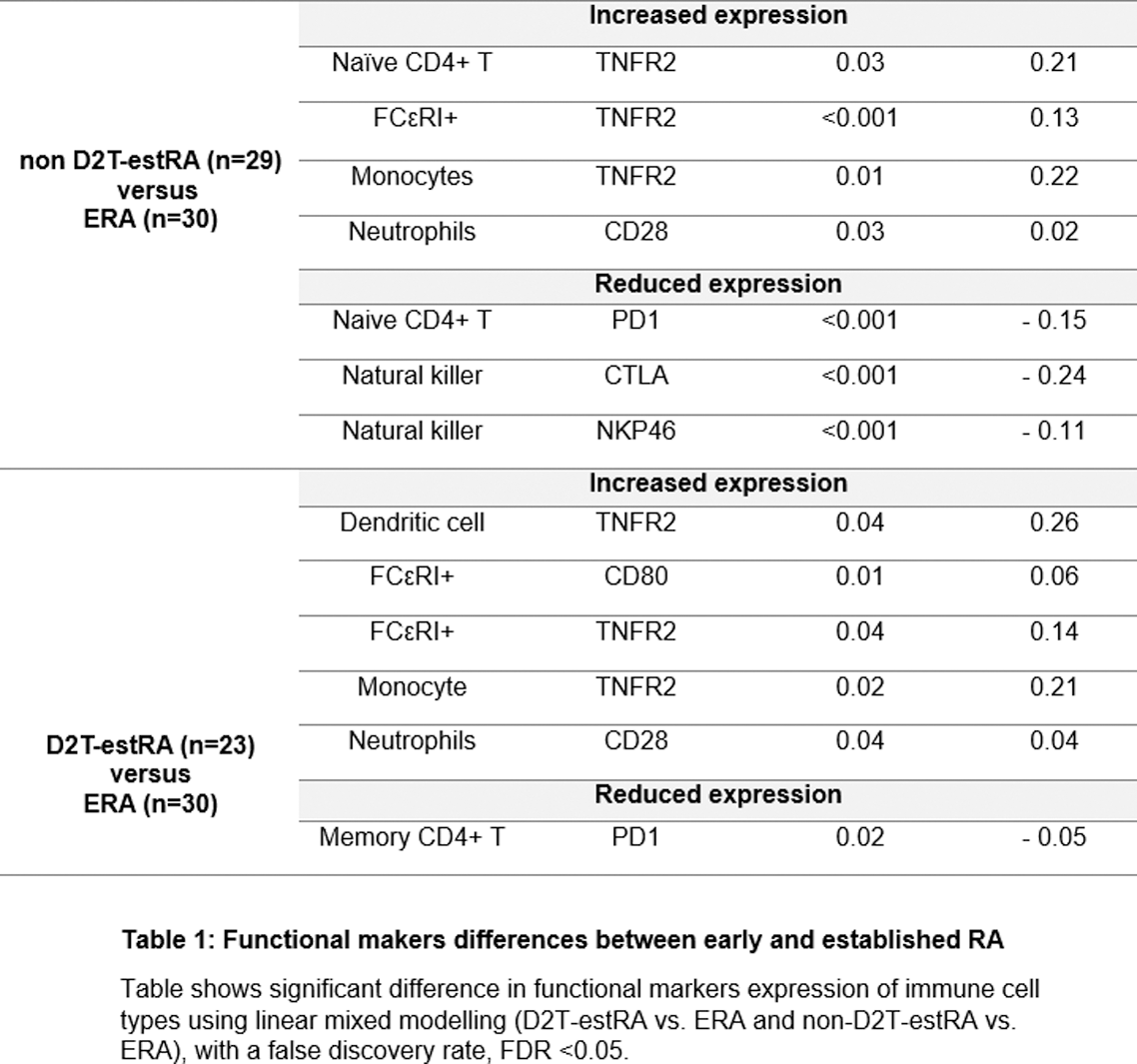
Results: The RA cohort was predominantly female (75/101, 73.3%) with a median age of 60 years (IQR 51- 65 years). 73/82 (89%) of RA patients were seropositive. Among those with D2T-RA, the median number of failed b/tsDMARDs was 3 (range 2-7). Both ERA and estRA had the same median disease activity scores-28 ESR (DAS28 ESR) and disease activity scores-28 CRP (DAS28 CRP), measuring 4.70 and 4.85, respectively. A total of 16 phenotypically distinct immune cell subsets were identified in both healthy individuals and RA patients (Figure 1). RA patients showed a lower proportion of FceR compared to healthy individuals. Further analysis revealed 50 significant functional differences across 13 distinct cell subpopulations between healthy individuals and those with RA. All subgroups of RA (ERA, non D2T-estRA, and D2T-estRA) exhibited similar abundance of immune cell subpopulations. However, significant differences in functional markers were observed within these cell subpopulations (refer to Table 1). In comparison to ERA, both estRA subgroups demonstrated elevated expression of TNFR2 (in monocytes and FCεRI+ cells) and CD28 (in neutrophils). Conversely, PD1 expression was lower in naïve CD4 T cells in non D2T-estRA and lower in memory CD4+ T cells in D2T-estRA. In addition, higher TNFR expression (naïve CD4+ T cells) and lower CTLA4 (natural killer cells) and NKP46 (natural killer cells) was observed in non D2T-estRA vs ERA. When comparing D2T-estRA to ERA, higher TNFR expression was observed in dendritic cells, along with increased CD80 expression in FCεRI+ cells.
Conclusion: These findings demonstrate dynamic immunological properties across RA stages. These may offer insights into the heterogeneous treatment responses and outcomes observed in clinical practice and provide a basis for further investigation.
REFERENCES: [1] Nagy, G. et al. Annals of the Rheumatic Diseases 80, 31 (2021).
Acknowledgements: This study was supported by a UCB pharma PhD studentship (TB).
Disclosure of Interests: None declared.