

Background: Povetacicept (ALPN-303; TACI vTD-Fc)[1] is an enhanced, high affinity dual antagonist of BAFF (B cell activating factor) and APRIL (a proliferation inducing ligand), cytokines that play key roles in the pathogenesis of multiple autoimmune diseases via their roles in the activation, differentiation, and/or survival of B cells, including antibody-secreting cells, as well as T lymphocytes and innate immune cells. Other therapeutic agents targeting BAFF and/or APRIL, including wild-type (WT) TACI-Fc fusion proteins, have demonstrated promising clinical potential in systemic lupus erythematosus (SLE), lupus nephritis, and other autoimmune diseases, but have generally targeted only BAFF, only APRIL, or BAFF more effectively than APRIL.
Objectives: 1.To investigate the impact of povetacicept on gene expression in human peripheral blood mononuclear cells (PBMC) stimulated in vitro with BAFF and/or APRIL.
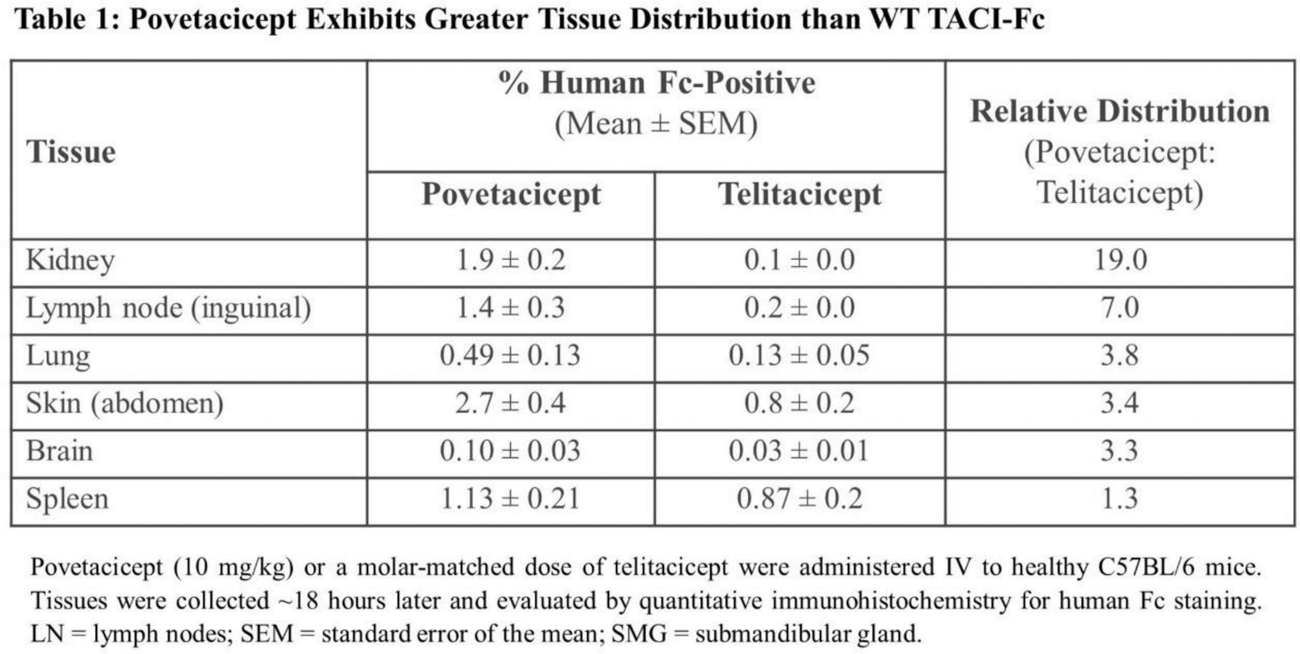
2.To compare the tissue distribution of povetacicept following a single dose to WT TACI-Fc (telitacicept) in adult mice.
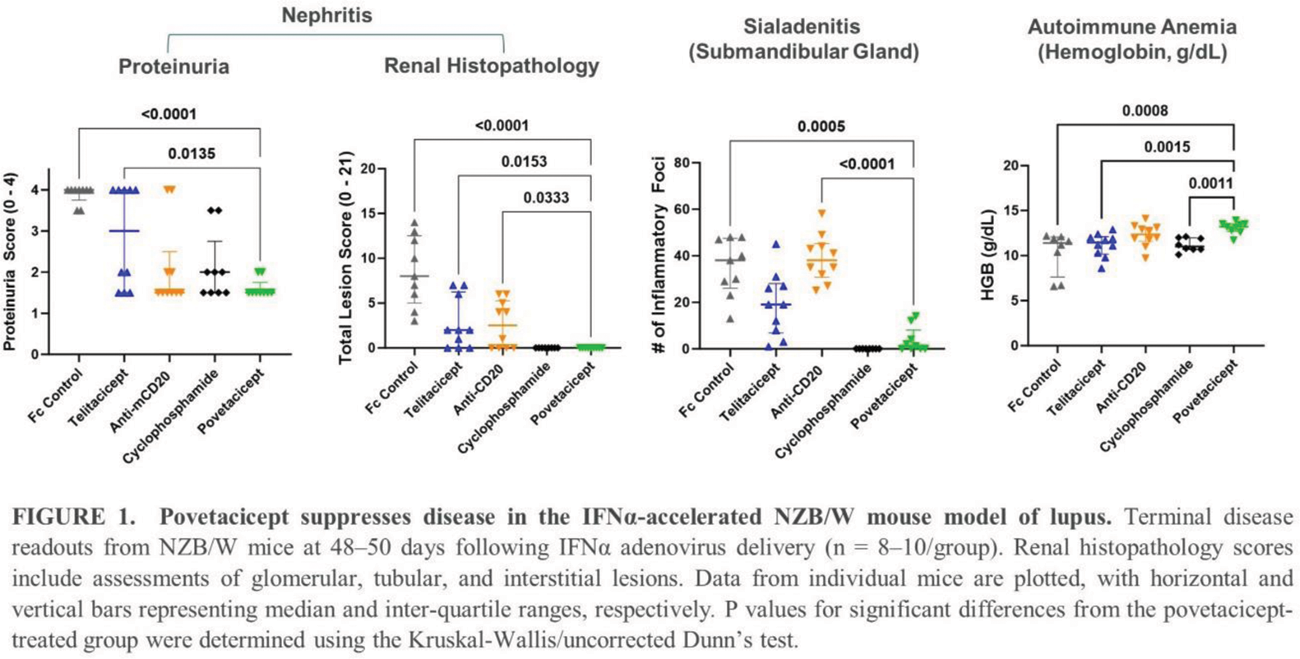
3.To evaluate the relative efficacy of povetacicept, telitacicept, and anti-CD20 in an accelerated NZB/W mouse model of lupus.
Methods: Publicly available RNA Sequencing (RNA-Seq) datasets from healthy donors (HD) and SLE patients were utilized to assess APRIL and BAFF gene expression.[2-4] HD PBMC were stimulated in vitro with 1 nM CD40L and 50 ng/mL IL-21 and cultured for 24 hours with 10 nM APRIL and 25 nM BAFF in the presence of either 50 nM of Fc control, povetacicept, anti-BAFF mAb (belimumab), or anti-APRIL mAb. Cultured cells were harvested for flow cytometry analysis or processed for bulk RNA-seq, and supernatants were assessed for cytokine production. To evaluate tissue distribution of povetacicept (10 mg/kg) as compared to telitacicept, various organs were collected ~18 hours following a single molar matched intravenous (IV) injection of test article to healthy C57BL/6 mice. Tissues were evaluated by quantitative immunohistochemistry for human Fc staining. In the IFNα-accelerated NZB/W lupus mouse model[5], 11-week-old female NZB/W mice were administered twice weekly intraperitoneal injections of povetacicept (10 mg/kg), a molar-matched dose of an Fc control or telitacicept, or weekly cyclophosphamide or a depleting anti-mouse CD20 mAb. Mice were analyzed 7 weeks later for disease endpoints, including proteinuria, nephritis, and anemia.
Results: In publicly available transcriptional datasets, PBMC from SLE patients exhibit a significant elevation of BAFF and/or APRIL compared to healthy adults. In CD40L/IL-21/BAFF/APRIL-stimulated PBMC, povetacicept suppressed multiple inflammatory pathways, particularly those associated with B cell activation and immunoglobulin production. Povetacicept also inhibited IFN signaling ( IFNGR1 , IFNGR2 ) and the Th17 activation pathway ( IL21 , IL6 , and IL10 ). In adult mice, povetacicept demonstrated greater distribution than telitacicept in many tissues, including lupus-related organs such as the kidney, skin, and brain ( Table 1 ). In the accelerated NZB/W lupus model, povetacicept significantly suppressed multiple disease parameters ( Figure 1 ) and reduced key disease-relevant immune cell subsets (including germinal center B cells and plasma cells) in the spleen more effectively than treatment with telitacicept or anti-CD20.
Conclusion: Dual inhibition of both BAFF and APRIL may be required to achieve optimal suppression of pathogenic pathways associated with SLE and related diseases. With improved affinity and enhanced tissue distribution over WT TACI-Fc, povetacicept may enable optimal BAFF/APRIL inhibition in SLE, as well as in other B cell- and/or autoantibody-related diseases. Clinical studies of povetacicept in autoimmune glomerulonephritis (NCT05732402) and cytopenias (NCT05757570) are ongoing; a Phase 2 study in SLE is in preparation.
REFERENCES: [1] Evans L, et al. 2023. Arthritis Rheumatol. 75(7):1187-1202.
[2] Ota M, et al. 2021. Cell. 184(11):3006-21.
[3] Banchereau, R, et al. 2016. Cell. 165(6):1548-50.
[4] Chiche L, et al. 2014. Arthritis Rheumatol. 66(6):1583-95.
[5] Liu Z, et al. 2011. Arthritis Rheumatol. 63(1):219-29.
Acknowledgements: NIL.
Disclosure of Interests: Katherine E. Lewis Alpine Immune Sciences, Inc., Alpine Immune Sciences, Inc., Zahra Maria Alpine Immune Sciences, Inc., Alpine Immune Sciences, Inc., Elizabeth Repash Alpine Immune Sciences, Inc., Alpine Immune Sciences, Inc., Tiffany Blair Alpine Immune Sciences, Inc., Alpine Immune Sciences, Inc., Amanda Enstrom Alpine Immune Sciences, Inc., Alpine Immune Sciences, Inc., Michelle A. Seaberg Alpine Immune Sciences, Inc., Alpine Immune Sciences, Inc., NingXin Wang Alpine Immune Sciences, Inc., Alpine Immune Sciences, Inc., Stanford L. Peng Alpine Immune Sciences, Inc., Alpine Immune Sciences, Inc., Stacey R. Dillon Alpine Immune Sciences, Inc., Alpine Immune Sciences, Inc.