

Background: Rheumatoid arthritis (RA) is characterised by synovial inflammation with CD4 + T cells playing a central role in the autoimmune process. Conversely, spondyloarthropathies are believed to be driven by CD8 + T cells. Pathogenic T cells within affected tissues usually exhibit limited T cell receptor (TCR) diversity and express activation markers. Several CD4 + T cell subsets and states have been identified in RA, but their immune phenotype, pathogenic potential and disease specificity has not been fully characterised, preventing their use as diagnostic markers in a clinical setting.
Objectives: Investigate the frequency, activation, TCR diversity, and disease specificity of various CD3 + T cell subsets in inflammatory arthritis, including HLA-DR + CD27 - CD4 + T EM [1] and PD-1 high CXCR5 - CD4 + T cells (Tph) [2] reportedly associated with RA.
Methods: Using 13-colour flow cytometry and the IOTest® Beta Repertoire Kit, peripheral blood mononuclear cells and matched synovial fluid cells from patients with five different rheumatic diseases (seropositive RA, seronegative RA, psoriatic arthritis, other spondyloathropathies, non-inflammatory controls including osteoarthritis) were immunophenotyped based on the expression of T cell markers and of 24 different TCR Variable Beta chains.
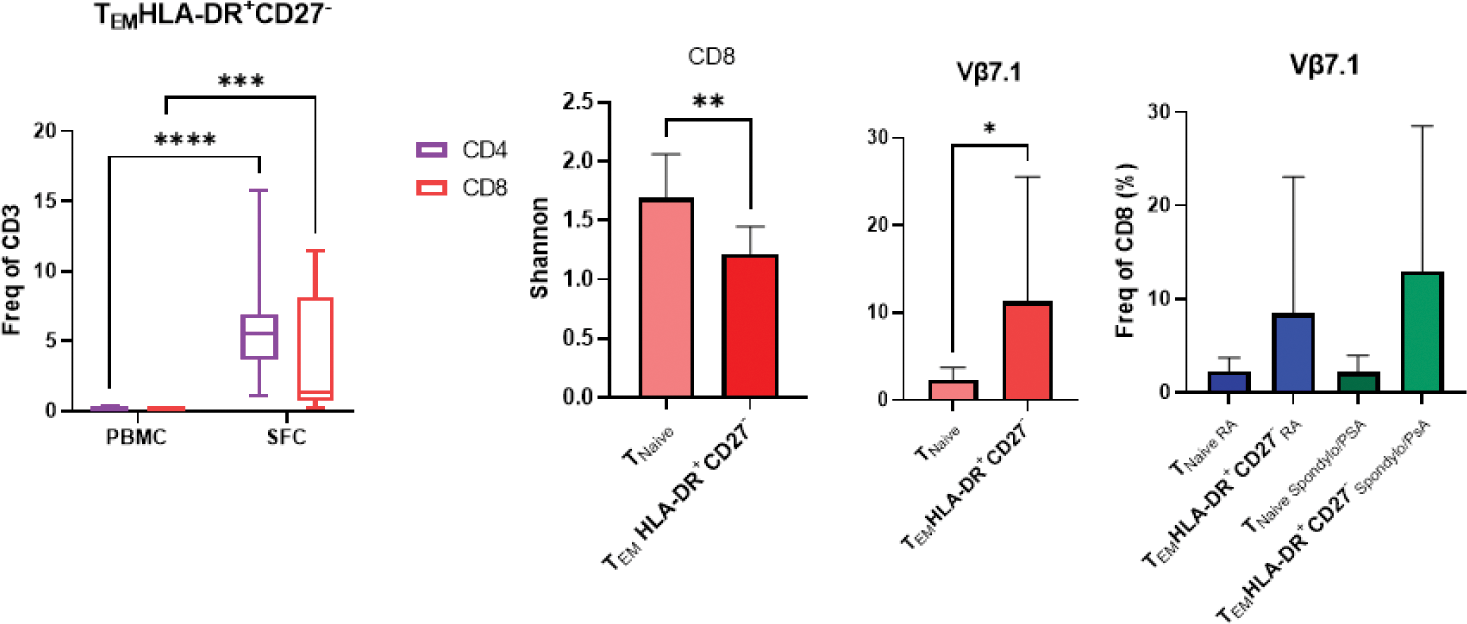
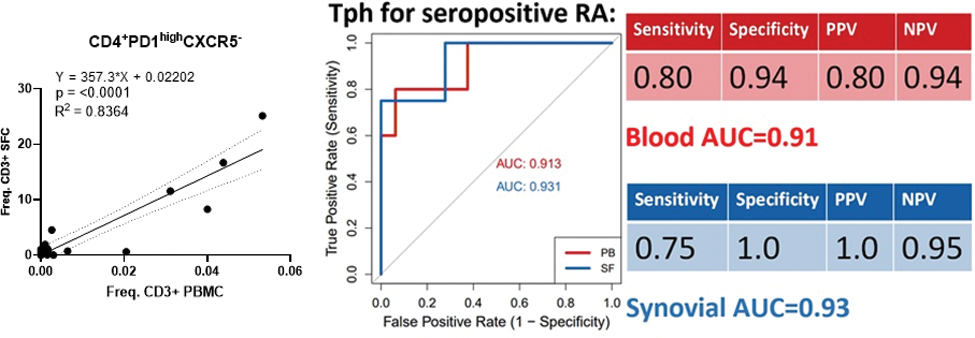
Results: Synovial fluid exhibits a significant enrichment of CD8 + over CD4 + T cells (p<0.03), regardless of disease classification. The CD8 + TCR repertoire is less diverse (lower Shannon diversity index, p<0.01), and the frequency of CD8 + T EM correlated strongly between blood and synovial fluid (p<0.006), supporting a pathogenic role for CD8 + T cells across all types of inflammatory arthritis. In general, immune cell types and cell states highly enriched in synovial fluid were not disease-specific but shared across diagnoses; they expressed markers of activation and differentiation; had a restricted TCR repertoire and their frequency within synovial fluid was correlated with their frequency in the blood. For example, HLA-DR + CD27 - CD4 + T EM [1] are 44.9 times more frequent in synovial fluid than in the blood (p<0.0001), but they are not specific to RA. They are a general biological feature of synovial inflammation. Interestingly, this is also true for their CD8 + counterpart (HLA-DR + CD27 - CD8 + T EM , relative frequency 72.6, p<0.001). Both subsets display a restricted TCR repertoire (decreased Shannon diversity index, p<0.01 and <0.001, respectively). In HLA-DR + CD27 - CD8 + T EM , the decreased TCR diversity is partly driven by an expansion Vβ7.1 + cells (relative frequency=5, p<0.01), but this expansion is not disease-specific ( Figure 1 ). In contrast, Tph were almost exclusively found in seropositive RA (Positive Predictive Value= 100%; Specificity= 100%; Negative Predictive Value= 90%; Sensitivity= 67%). The Shannon diversity index is significantly decreased in Tph cells (p<0.05), with decreased frequency of Vβ1 and Vβ5.2 and expansion of Vβ9 (p<0.01, p<0.05 and p<0.05 respectively). In addition, Tph frequencies show correlations with disease activity and autoantibody titres. Although Tph frequency is >350 higher in synovial fluid (p<0.001) compared to blood, frequencies in both compartments were strongly correlated (R 2 =0.84 and p<0.0001), which implies an identical predictive accuracy in these two anatomical compartments (AUC > 0.91, Figure 2 ). This is a proof of principle that blood immune cell types have a strong diagnostic potential. Based on these observations, the detection of Tph cells in one of our seronegative patient prompted a clinical reassessment, resulting in a re-classification to seropositive RA (Tph in synovial fluid pre-dated seroconversion).
Conclusion: We demonstrate the potential of an experimental setting to infer immune cell pathogenicity in inflammatory arthritis and establish the proof of principle to identify diagnostic signatures from peripheral blood.
REFERENCES: [1] Fonseka, Sci Transl Med, 2018; [2] Rao, Nature, 2017.
Acknowledgements: NIL.
Disclosure of Interests: None declared.