

Background: In systemic AA amyloidosis (AAA) the amyloid fibrils are derived from serum Amyloid A protein (SAA) and potential underlying causes include almost any condition that can induce persistent systemic inflammation. AAA presents with proteinuria, followed by progressive renal impairment and involvement of other organs. Current treatment relies on identification of the underlying disease and therapy aimed at sustained suppression of the inflammatory response.
Objectives: To analyse the change in inflammatory disease underlying AAA in cases seen the UK national referral centre over 34 years.
Methods: All new patients seen in a UK national referral centre with AAA were included. The medical records were reviewed up until December 2023 for demographics, histology, and underlying inflammatory disorder. Underlying diagnosis was classified based on the opinion of the MDT at the time of assessment. Patients were divided into 6 eras based on when they were initially reviewed: pre 2000 (190 cases), 2001-04 (142 cases), 2005-09 (188 cases), 2010-14 (189 cases), 2015-19 (156 cases), 2020 onwards (89 cases).
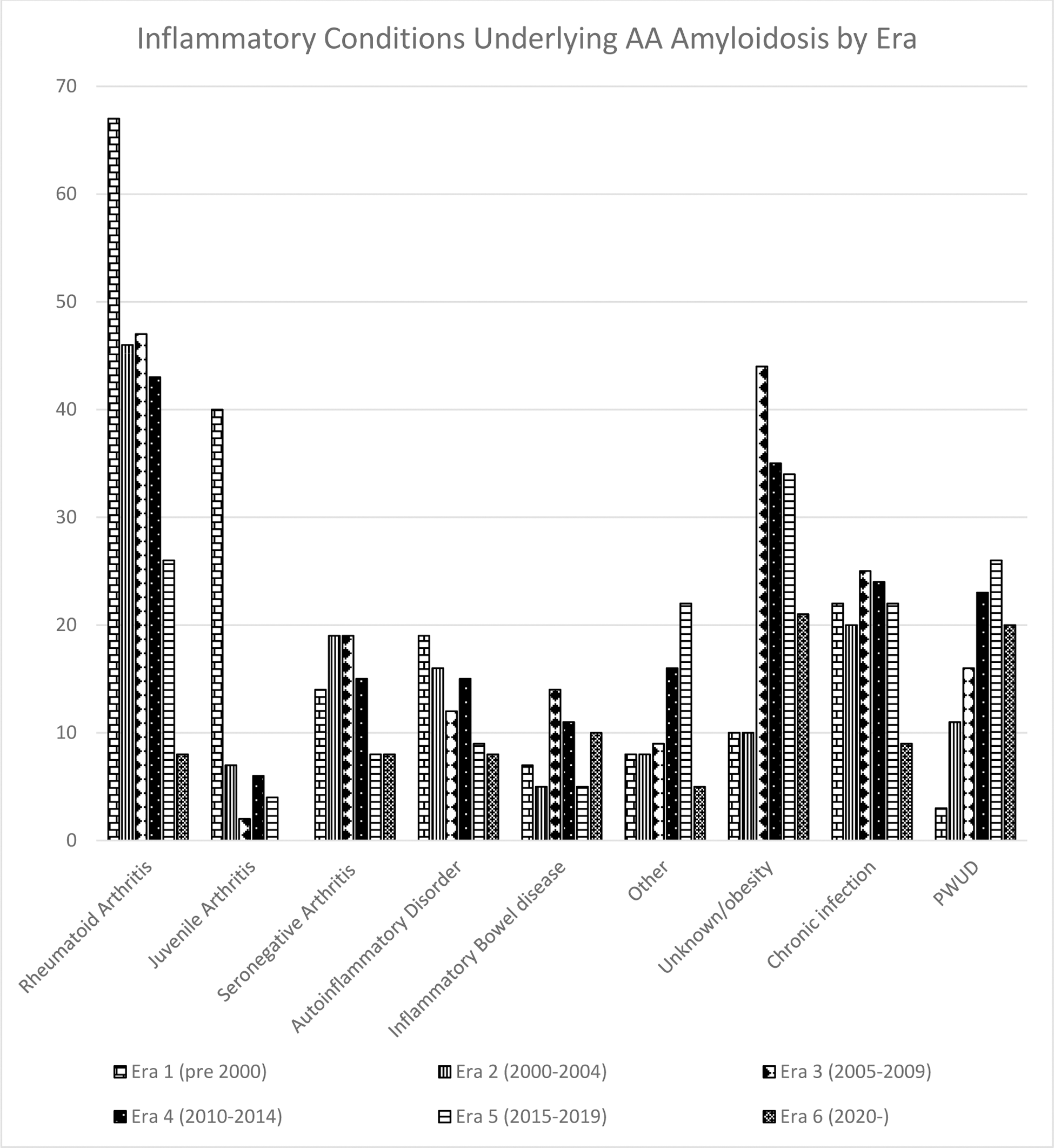
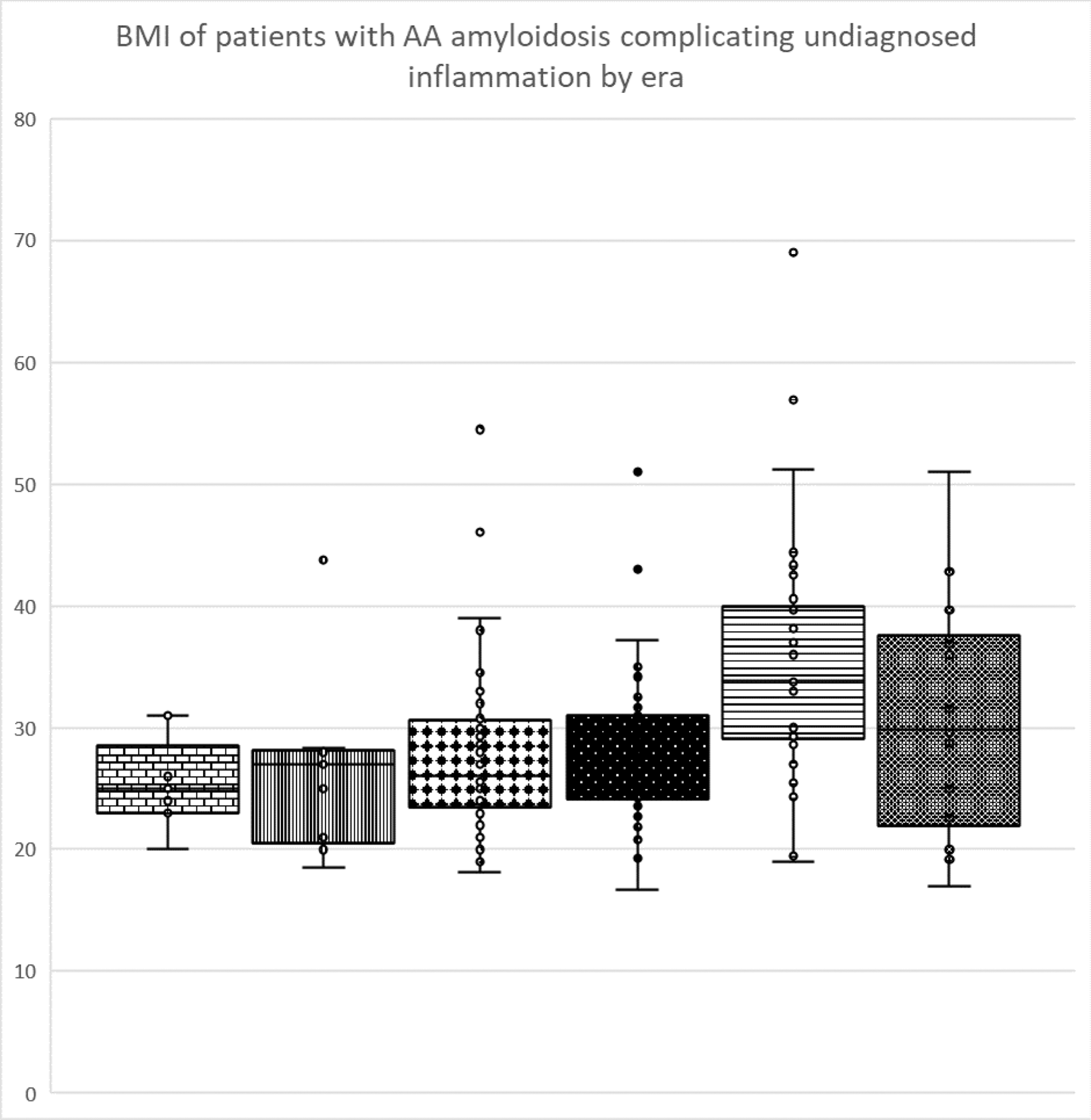
Results: Patients: 952 patients with AAA were seen between Jan 1990 and December 2023; 492 (52%) were male. The age of patients at first review ranged from 9.5 to 91 (median 53.4) years. 78% were of White European, 11% South Asian, 6% West Asian/Arab, 5% Black African and 0.2 % East Asian descent. Diagnoses: 237 patients (25%) had rheumatoid arthritis, 59 (6%) juvenile inflammatory arthritis, 83 (9%) seronegative arthritis, 79 (8%) an autoinflammatory disorder, 52 (5%) inflammatory bowel disease, 68 (7%) other (of which: 29% malignancy, 17% unicentric Castleman’s disease, 14.5% vasculitis, 11.5 % genetic disorders - predominantly cystic fibrosis, 10% granulomatous disorders, 4% crystal arthropathy), unknown/obesity 154 (16%), chronic infection 122 (13%), complication of long term injected drugs in people who use drugs (PWUD) 99 (10%). Changes over time: AAA has fallen from 23.8% to 12.3% to 10.9%, to 6.5% to 3.6% to 2.3% of new amyloid cases across the 6 eras, and there has been significant changes in the age at diagnosis which has increased from a median of 48 years pre 2000 to 58 post 2020. Patients diagnosed in era 1 were significantly younger than in all of the following eras and those diagnosed in era 2 were younger than those diagnosed after 2010 (p<0.05). There have been significant reductions in the number of patients with underlying rheumatoid arthritis and juvenile inflammatory arthritis and increase in the numbers an unknown cause of underlying inflammation and in PWUD (p < 0.001) (Figure 1). Body mass index has risen over time in the unknown diagnosis group (p< 0.05) with 61% of patients diagnosed since 2015 having a BMI > 30 (Figure 2).
Conclusion: AAA has become rarer over time, in contrast to AL and hereditary amyloidosis which have remained consistent across the period. The change in distribution of underlying diseases appears to reflect the impact of biologics in successful treatment of rheumatoid arthritis and juvenile arthritis although there has been less effect in AAA complicating seronegative arthritis or inflammatory bowel disease. Since 2015 more patients presented with AAA complicating inflammation of unknown aetiology or obesity than any diagnosed chronic inflammatory disorder, followed by AAA in PWUD. As AAA is a late complication of chronic inflammation this increase may partially reflect better long term survival amongst users as well as increasing problem drug dependence. The rise in AAA complicating inflammation of unknown cause appears to be genuine. The increasing BMI in this group supports AAA as a rare complication of the low-grade inflammation resulting from adipocyte production of IL-6 which can be seen in some obese individuals.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Helen J. Lachmann SOBI, Novartis, Alexion-AZ, Oliver Cohen: None declared, Mariana Fontana Alynlam, Pfizer, Intellia, GSK, Janet Gilbertson: None declared, Julian D. Gillmore: None declared, Philip N Hawkins Alnylam, GSK, Shameem Mahmood: None declared, Ana Martinez Naharro: None declared, Claire Peet: None declared, Sriram Ravichandran: None declared, Dorota Rowczenio: None declared, Ashutosh D. Wechalekar Janssen, Attralus, Alexion, Janssen, Prothena, Pfizer, Alexion-AZ, GSK, Attralus, Carol Whelan Alnylam, Pfizer, Astrazeneca.